One of the greatest gemological challenges is determining if a ruby or sapphire has been heat treated. UV fluorescence can assist in that identification, as well as detecting fillers in emerald.
One of the greatest challenges gem dealers and gemologists face today is being able to accurately determine if a stone has been heat treated. While a 100% reliable answer to that question is a job for a major gem lab, there is a simple and inexpensive tool that can often give an important indication.
So what is this miracle tool? We speak of the lowly ultraviolet light.
It wasn’t long ago that ultraviolet (UV) fluorescence was considered the poor stepchild of the gem lab, a pint-sized pea shooter when compared with the high-caliber cannons available in modern labs. But with the rising importance of treatment detection, the humble UV lamp is making a comeback.
Reactive
Many heat-treated rubies and sapphires will display chalky short-wave (SW) fluorescence. This reaction is practically never found in untreated corundums and was first noted by Robert Crowningshield (1966, 1970). It is actually the colorless portions of the stone that fluoresce (a reaction similar to Verneuil synthetic sapphires). Since colorless areas follow the original crystal’s growth structure, the fluorescence will follow the same pattern as the gem’s color zoning. In addition, other trace elements in corundum may produce fluorescent reactions, from the well-known red glow of ruby to other reactions that are still not completely understood. Many will be illustrated below, both known and unknown.
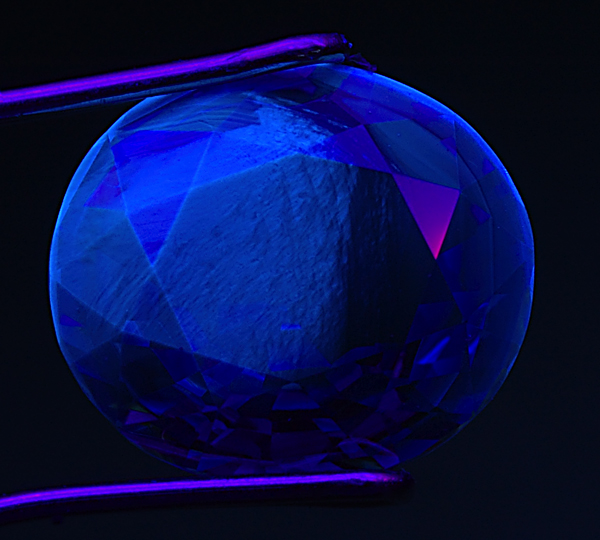 Figure 1. Tufted love
Figure 1. Tufted love
When a sapphire is subjected to high-temperature heat treatment, a chalky blue to blue-green SW fluorescence is often created. As seen above, this reaction is confined to certain zones in the gem. These “tufted” fluorescent zones follow the crystallographic structure of the gem. Photo: Richard W. Hughes; Nikon D70
 Figure 2. Ring around the collar
Figure 2. Ring around the collar
Flipping over the same sapphire from Figure 1 reveals a distinct bluish (‘chalky’) fluorescent ring, corresponding to the colorless portions of the gem when viewed in immersion. When seen, this strong chalky blue SW fluorescence is an extremely strong indication that the gem has been subjected to high-temperature heat treatment.
Photo: Richard W. Hughes; Nikon D70
 Figure 3. Another example of zoned chalky SW fluorescence in a heat-treated sapphire. Photo: Richard W. Hughes; Nikon D200
Figure 3. Another example of zoned chalky SW fluorescence in a heat-treated sapphire. Photo: Richard W. Hughes; Nikon D200
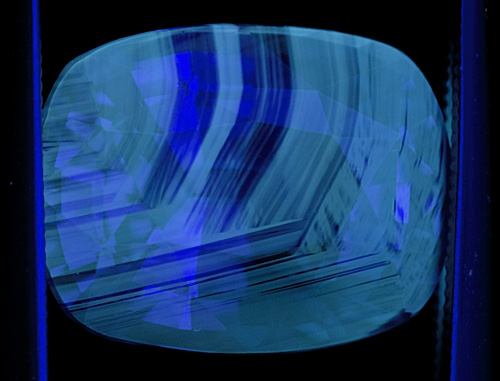 Figure 4. Another blue sapphire showing chalky fluorescence corresponding to the colorless portions of the gem. When seen, this strong chalky blue to green SW fluorescence is an extremely strong indication that the gem has been subjected to high-temperature heat treatment. Note that this fluorescence often appears in patterns that resemble the graining of wood. Photo: Richard W. Hughes; Nikon D200
Figure 4. Another blue sapphire showing chalky fluorescence corresponding to the colorless portions of the gem. When seen, this strong chalky blue to green SW fluorescence is an extremely strong indication that the gem has been subjected to high-temperature heat treatment. Note that this fluorescence often appears in patterns that resemble the graining of wood. Photo: Richard W. Hughes; Nikon D200
 Figure 5. Fluorescent fillers in emerald
Figure 5. Fluorescent fillers in emerald
UV fluorescence can also help identify treatments in emerald. Emeralds are typically enhanced by filling their fissures with oils/resins. Some of these fluoresce. In the photo above, an emerald is exposed to long-wave UV light. The filler in the fissures is clearly identified by its bluish fluorescence. Note that the body color of the stone appears much different under UV light.
Photo: Richard W. Hughes; Nikon D70
Show and Tell
So how does one go about checking for this reaction? The first step is to obtain a combination LW/SW lamp. You will also need a pair of protective glasses (SW light can burn your eyes with prolonged exposure). A viewing cabinet is also a plus. Finally, you will need a small lens to magnify the stone. Figures 6 –8 show one setup for both viewing and photographing fluorescence.
When observing fluorescence, the idea is to hold the stone with tweezers and bring it as close as possible to the lamp and view it under magnification. Examine the stone from all angles; many times the key chalky areas are confined to tiny portions of the stone.
One of the authors (Hughes, 1997) has suggested a lens be incorporated as an integral part of the viewing cabinet, but sadly instrument manufacturers have yet to produce such a unit.
 Figure 6. Setup for close-up examination of UV fluorescence.
Figure 6. Setup for close-up examination of UV fluorescence.
A small piece of blutac or clay inserted between the UV lamp and the viewing cabinet creates a small gap. This allows the stone to be viewed while positioned extremely close to the lamp, greatly increasing the ability to catch weak reactions. A lens is positioned to magnify the stone during viewing. The masking tape keeps the lamp from tipping off the cabinet. Special UV protection glasses such as those in front of the cabinet should be worn to protect the eyes from harmful SW radiation. Photo: Richard W. Hughes; Nikon D70
 Figure 7. Photographing fluorescence
Figure 7. Photographing fluorescence
Some photos in this article were taken using the following crude set-up. A stoneholder was taped to a gooseneck arm to hold the stone in place. Photos were shot with a tripod-mounted Nikon D70 digital camera with a Nikon 60mm 2.8 macro lens. Also finding sometime use were two Nikon screw-on close-up lenses, to further increase magnification. Exposures were made in manual mode with a wireless shutter release, with all ambient (room) lights turned off. Exposures ranged from a few seconds to over half a minute. Later, the setup was refined to a tripod-mounted Nikon D200 and the aforementioned 60mm 2.8 macro lens. In a darkened room, with the UV lamp hand-held, some spectacular shots were obtained. Photo: Richard Hughes
 Figure 8. Another view
Figure 8. Another view
Another view of the photo setup. Photo: Richard Hughes
Caveats
This test does require a bit of knowledge. If a ruby or sapphire shows a chalky fluorescence in SW, it is probably heat treated. If it is inert, that does not mean it’s unheated. Also be careful that the stone is clean. Soap and other chemicals can also produce chalky fluorescence. And while this test is a tool that can be extremely useful, it is not a substitute for a complete gemological examination in a fully-equipped laboratory. Finally, keep the exposure times of corundum to SW fluorescence to a minimum. SW irradiation does create a yellow color center that can alter the color of the gem; even five minutes exposure can do this (see Figure 9). While this color fades with prolonged exposure to daylight, it can turn a blue stone more greenish (not good if it’s your stone and you’re trying to sell it).
 |
 |
| Figure 9. At left is a blue sapphire in a ring; at right, the same stone following a few minutes irradiation by SW UV. This yellow color will fade with exposure to sunlight, but illustrates how one should not expose corundums for prolonged periods to SW UV. Photos: Richard Hughes |
|
One further caveat concerns a type of chalky green SW fluorescence sometimes seen in natural, untreated blue sapphires (particularly those from Madagascar). The fluorescence tends to be weak, and extremely superficial, being limited to thin layers at the surface. In addition, the fluorescent patches tend to have sharper boundaries than the reaction in heated stones (Figures 10–12).
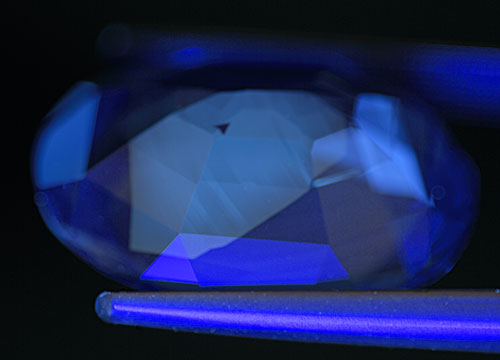 Figure 10. Occasionally we see a chalky green SW fluorescence in untreated natural sapphires, particularly those from Madagascar. This fluorescence tends to be restricted to a thin layer at the surface of the stone and has sharp boundaries, as shown above. The small black triangular area is simply a non-fluorescent zone. Photo: Richard W. Hughes; Nikon D200
Figure 10. Occasionally we see a chalky green SW fluorescence in untreated natural sapphires, particularly those from Madagascar. This fluorescence tends to be restricted to a thin layer at the surface of the stone and has sharp boundaries, as shown above. The small black triangular area is simply a non-fluorescent zone. Photo: Richard W. Hughes; Nikon D200
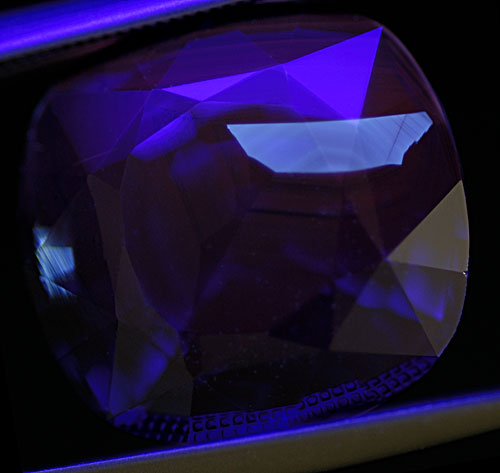
 Figure 11. Distant and magified views of another superficial fluorescent patch in an untreated sapphire from Madagascar.
Figure 11. Distant and magified views of another superficial fluorescent patch in an untreated sapphire from Madagascar.
Photos: Richard W. Hughes; Nikon D200
 Figure 12. Using fiber-optic illumination with the microscope, the fluorescent patch in the stone from Figure 11 is revealed as a clear area without texture clouds. Photo: Richard W. Hughes; Nikon D200
Figure 12. Using fiber-optic illumination with the microscope, the fluorescent patch in the stone from Figure 11 is revealed as a clear area without texture clouds. Photo: Richard W. Hughes; Nikon D200
Breaking Down Fluorescence
In its most basic sense, fluorescence is the emission of visible energy of a longer wavelength when bombarded by energy of a shorter wavelength. The stimulating energy may be x-rays (x-ray fluorescence), ultraviolet light (UV fluorescence) or even visible light. Ruby provides an excellent example of the latter.
When a ruby is put into daylight, certain electrons are excited to higher orbitals, producing absorption of the corresponding wavelengths. But instead of falling straight back to the ground state, the electrons fall in steps. In most cases, the release of energy from each of those steps is in the form of phonons to the crystal lattice (vibrational heat), and thus invisible to the human eye. But in the case of ruby, some emissions fall into the red (at 692.8 and 694.2 nm). This is what makes ruby so special; not only does it possess a red body color, but that red body color is supercharged by red fluorescence. This is what led the ancients to believe ruby had a fire burning inside.
UV fluorescence can be an extremely sensitive indicator not only of trace impurities, but also the conditions under which the gem formed. Indeed, it is not unusual for fluorescence to be easily seen from strongly-fluorescing ions at concentrations in the range of 0.01 parts-per-million (ppm). For lay people, that’s an itsy-bitsy amount, completely beyond the detection limits of all but the most sophisticated and expensive analytical equipment.
 Figure 13. One of the remaining corundum mysteries is the cause of the "apricot" orange fluorescence seen in many sapphires of both blue and yellow color, particularly those from Sri Lanka and Madagascar. This fluorescence may be seen in both LW and SW, with LW always being stronger, and is unaffected by heat treatment. The above stone is an untreated Madagascar blue sapphire in LW, the same stone as shown in Figures 11 and 12. Note that the culet area, which contains the heaviest concentration of blue color, is inert. Photo: Richard W. Hughes; Nikon D200
Figure 13. One of the remaining corundum mysteries is the cause of the "apricot" orange fluorescence seen in many sapphires of both blue and yellow color, particularly those from Sri Lanka and Madagascar. This fluorescence may be seen in both LW and SW, with LW always being stronger, and is unaffected by heat treatment. The above stone is an untreated Madagascar blue sapphire in LW, the same stone as shown in Figures 11 and 12. Note that the culet area, which contains the heaviest concentration of blue color, is inert. Photo: Richard W. Hughes; Nikon D200
 Figure 14. Zoned "apricot" orange LW fluorescence in an untreated Madagascar blue sapphire. Photo: Richard W. Hughes; Nikon D200
Figure 14. Zoned "apricot" orange LW fluorescence in an untreated Madagascar blue sapphire. Photo: Richard W. Hughes; Nikon D200
Speaking of Sapphire
While the red fluorescence of ruby is detailed in many gemological texts (c.f. Hughes, 1997), the cause of the chalky fluorescence has not been covered. Let’s take a look at it.
Sapphire generally shows no fluorescence to visible light. But that changes if we expose it to short-wave UV. This is most clearly seen in synthetic colorless sapphire, which displays a bluish white (‘chalky’) emission in the range of 410–420 nm.
Synthetic sapphire
This blue fluorescence in synthetic sapphire has been observed at least since 1948. While it has been generally ignored in the gemological literature, it has been the subject of numerous scientific papers (c.f. Evans, 1994).
Evans surmised after reviewing the data that the 410–420 nm fluorescent peak was due to Ti4+ charge-transfer transition. That was later confirmed by Wong, et al. (1995a and 1995b). Isolated Ti4+ ions, or Ti–Al vacancy pairs produce this fluorescence.
The Ti4+ charge-transfer transition in corundum is so strong and the efficiency so high that the fluorescence is easily observed by eye at even just 1 ppm Ti4+. Most of the synthetic sapphire in the market contains at least one ppm of Ti4+ from the Al2O3 starting material, if not more, and thus fluoresces. The fluorescence peaks at about 415 nm at very low Ti4+ concentrations, but as the concentration increases, the fluorescent band broadens and the peak shifts to as high as 460 or 480 nm, making the fluorescence appear more greenish-blue or whitish-blue.
Why this chalky fluorescence occurs relates to the growth temperature and Ti4+ concentrations relative to other impurities. In synthetic corundums, the high growth temperatures and high Ti4+ concentrations produce the chalky fluorescence. In certain heat-treated sapphires with low Fe levels (such as those from Sri Lanka), high-temperature heat treatment creates similar conditions to the synthetic. Thus the chalky fluorescence.
Natural sapphire
But what about natural, untreated sapphires? Why don’t they fluoresce blue or bluish white? The reason relates to growth temperatures and time. Natural sapphires grow at much lower temperatures, so Ti4+ is much less likely to pair up with Al vacancies.
These lower temperatures also allow easier pairing of Ti4+ with other ions (usually Fe2+ or Mg2+) that prevent fluorescence. Another damper is the presence of Fe3+, which also kills fluorescence. And finally, as the crystal sits in the ground for millions of years, diffusion slowly takes place, allowing the Ti4+ to slowly pair up with other ions, thus killing the fluorescence.
Heat-treated sapphire
Why then, do some heat-treated blue sapphires fluoresce chalky blue to green or white, and what causes the difference in appearance?
When blue (or geuda) sapphires are found in nature, they usually contain exsolved rutile. Titanium is concentrated in these rutile micro-crystals. When the stone is heat treated, the rutile dissolves into the corundum by diffusion, but because diffusion is slow, the local concentration of Ti4+ can be quite high. In the high concentration regions the Ti4+ concentration will exceed the local charge compensators (Fe2+ or Mg2+) and thus free Ti4+ ions will form. In addition, the dissolution of rutile will locally force the creation of some aluminum vacancies and some of the Ti4+–Al vacancy clusters will form. These types will fluoresce and thus some heat-treated sapphire will fluoresce somewhat like synthetic Ti-bearing sapphire. Because the original distribution of rutile (and iron in solution) occurred in zones, the distribution of the fluorescence will reflect that zoning. The fluorescence will be most intense where Fe is lowest and Ti4+ is highest, i.e. in areas of minimal color. The high iron-content basaltic sapphire (such as that from Australia, Thailand, etc.) will not fluoresce after heat treatment, as the iron concentration is much higher than the Ti4+ concentration everywhere.
All of the above is summarized in this table:
| Material | Impurity Levels | Growth Temperature | Growth Speed | Chalky SW UV Fluorescence |
|---|---|---|---|---|
| Synthetic sapphire | Low | High | Fast | Chalky |
| Natural sapphire | Various | Low | Slow | Inert |
| Heat-treated sapphire (low Fe type) | Hi Ti relative to Fe | Initially low; high during treatment | Slow; fast during treatment | Chalky in zones |
| Heat-treated sapphire (Fe-rich type) | Low Ti relative to Fe | Initially low; high during treatment | Slow; fast during treatment | Inert |
Superficial
The appearance of chalky fluorescence in a corundum depends strongly on both the Ti4+ and Fe3+ concentrations. Considering Ti4+ first, it is important to note that the charge-transfer absorption in the UV per ion is extremely high. If we look at the fluorescence of a piece of synthetic sapphire with several ppm of Ti4+, it seems to glow blue throughout the volume. This is because the total Ti4+ charge-transfer absorption is low enough that the UV photons can penetrate into the bulk of the sample. When the Ti4+ concentration is higher, the fluorescence seems to be coming from a thick layer near the surface because that is as far as the UV photons can penetrate. At high Ti4+ concentrations, only a thin surface layer is penetrated by the UV and the fluorescence appears as a chalky surface layer (see Figures 15 & 16). The charge transfer absorption of Fe3+ is also very high. Thus iron will contribute to limiting the penetration of UV into the sample also. Thus the very different appearance of the fluorescence of some synthetic sapphire and some heat-treated natural sapphire is not a different phenomenon, just a difference in impurity concentration.
One of the authors (JLE) has used a Schott BG-12 filter to heighten the superficial chalky fluorescence often seen in heat-treated ruby. This filter eliminates the red fluorescence and transmits the Ti4+ blue fluorescence (Figure 17).
 Figure 15. Let it glow
Figure 15. Let it glow
In this heat-treated and flux-healed ruby from Möng Hsu, Burma, thin zoned patches of chalky blue fluorescence float on top of the chromium-based red body fluorescence. These chalky blue zones are a strong indication of high-temperature heat treatment. SW UV.
Photo: Richard W. Hughes; Nikon D70
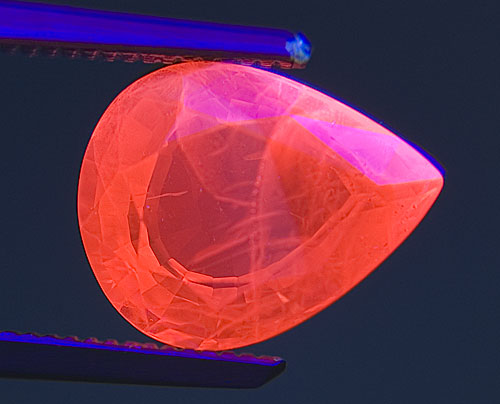 Figure 16. Going without
Figure 16. Going without
Another heat-treated and flux-healed ruby from Möng Hsu, Burma. Thin, zoned patches of chalky blue fluorescence float atop the chromium-based red body fluorescence. These chalky blue zones are a strong indication of high-temperature heat treatment, but are masked by the red fluorescence and therefore difficult to see. SW UV. Photo: Richard W. Hughes; Nikon D70
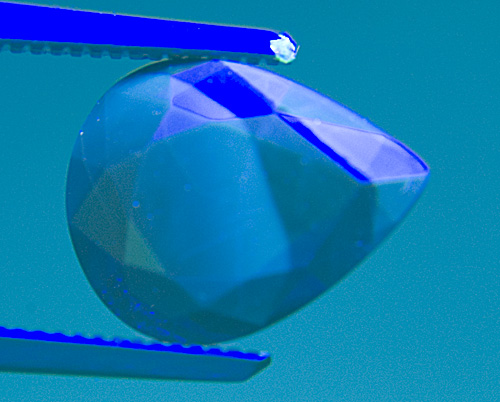 Figure 17. It’s easy being green
Figure 17. It’s easy being green
In the same stone from Figure 16, a green Schott BG-12 filter is placed over the gem. It removes the red fluorescence, thus making the chalky blue areas far easier to see. SW UV. Photo: Richard W. Hughes; Nikon D70
Traditional Gemologist Versus the Laser Junkie
While laser junkies focus strongly on the subject of fluorescence of ions in crystals, it is an orphaned topic in gemology. Let’s look at the different approaches.
With gemology, fluorescence is typically only visually observed with either LW or SW UV radiation, with the results recorded in terms of just brightness, color, and the presence or absence of phosphorescence.
In the study of ions in crystals, the parameters measured are more extensive. Typically the spectral distribution of the fluorescence is measured, as well as the spectral distribution of the light that can excite that fluorescence (excitation spectrum). In addition, the temporal decay parameters of the fluorescence are measured using a short pulse light source. Sometimes the decay curve is a single exponential indicating a single site or a single ion. Other times the decay curve is a combination of two or more exponentials indicating multiple sites or multiple ions. All of these parameters are often measured as a function of temperature.
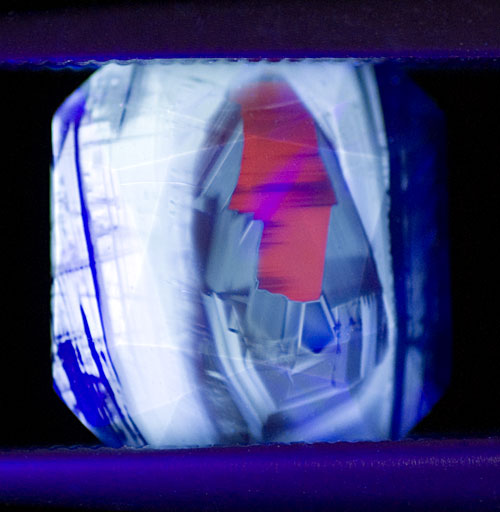 Figure 18. The myth of purity
Figure 18. The myth of purity
Crystals arise not from an ideal source, but spread out from a mixed broth. As they grow, the composition of that nectar changes because each influences the other. Diffusion is always a two-way street. The above photo is a map-like microcosm of this concept. Compositional areas lie tightly bounded in places, while blurred in others. The notion that either terrestrial or biologic creations might be "pure" is a myth. All are products of the past, all are affected by the present, all will be affected by the environment in which they reside, while simultaneously affecting that environment themselves. Each of these conditions is unique to the individual, as the above image shows. Thus no two will ever be alike. Heat-treated blue sapphire in SW UV. Photo: Richard W. Hughes; Nikon D200
Summing Up
With UV fluorescence, we have something all too rare in gemology today: an inexpensive test that is as sensitive as even bomb-science level analytical equipment.
Now what does this mean for a gem dealer? With a small UV lamp, one can quickly check potential purchases. Any stones that show a chalky SW fluorescence are most likely heat treated. Total equipment outlay? The lamp alone costs less than $300. Heh, heh, heh, we can already see you smiling.
And for the laboratory gemologist? This technique has been under-appreciated in the gemological community. Fluorescence might provide an avenue to determine if some sapphires have received heat treatment at temperatures lower than those normally used for geuda. But this will require adopting some of the techniques and instrumentation of the laser junkie. While expenditures for such instrumentation are hard to justify without a guarantee of just what may be learned, the increasing need to stay abreast of gemstone treatments requires an expansion of our array of techniques. Sophisticated fluorescence instrumentation is far less expensive than SIMS, LA-ICP-MS or LIBS analysis. Perhaps not quite so sexy (nor nearly as expensive), but when it comes to utility, this still looks like a pretty decent dance partner.

About the authors
Richard W. Hughes is one of the world’s foremost experts on ruby and sapphire. The author of many books and over 170 articles, his writings and photographs have appeared in a diverse range of publications, and he has received numerous industry awards. Co-winner of the 2004 Edward J. Gübelin Most Valuable Article Award from Gems & Gemology magazine, the following year he was awarded a Richard T. Liddicoat Journalism Award from the American Gem Society. In 2010, he received the Antonio C. Bonanno Award for Excellence in Gemology from the Accredited Gemologists Association. The Association Française de Gemmologie (AFG) in 2013 named Richard as one of the Fifty most important figures that have shaped the history of gems since antiquity. In 2016, Richard was awarded a visiting professorship at Shanghai's Tongji University. 2017 saw the publication of Richard and his wife and daughter's Ruby & Sapphire • A Gemologist's Guide, arguably the most complete book ever published on a single gem species and the culmination of four decades of work in gemology. In 2018, Richard was named Photographer of the Year by the Gem-A, recognizing his photo of a jade-trading market in China, while in 2020, he was elected to the board of directors of the Accredited Gemologists Association and was appointed to the editorial review board of Gems & Gemology and The Australian Gemmologist magazine. In 2022, Richard published Jade • A Gemologist's Guide, while 2024 brought Broken Bangle • The Blunder-Besmirched History of Jade Nomenclature. His jade trilogy was completed in 2025 with his translation of Heinrich Fischer's Nephrite and Jadeite.
Dr. John Emmett is one of the world's foremost authorities on the heat treatment, physics, and chemistry of corundum. He is a former associate director of Lawrence Livermore National Laboratory and a co-founder of Crystal Chemistry, which is involved with heat treatment of gemstones.
Acknowledgements
RWH wishes to thank John I. Koivula for his encouragement and suggestions during the writing of this article.
Notes
This article came about following RWH's plunge back into serious gemology in January 2005, when he joined the AGTA GTC. While checking the SW fluorescence of a heated sapphire, he decided to call JLE to inquire about the cause of the chalky fluorescence in heated and synthetic sapphires. "Interesting that you should ask," Emmett replied. "I've been doing much thinking about that same subject of late." And so it was that RWH and JLE began sharing thoughts on this subject.
Penned in bits and pieces in the first half of 2005; an edited version appeared in The Guide (Sept.–Oct. 2005, Vol. 24, Issue 5, Part 1, pp. 1, 4–7.
References
- Crowningshield, R. (1966) Developments and Highlights at the Gem Trade Lab in New York: Unusual items encountered [sapphire with unusual fluorescence]. Gems and Gemology, Vol. 12, No. 3, Fall, p. 73.
- Crowningshield, R. (1970) Developments and Highlights at GIA’s Lab in New York: Unusual fluorescence. Gems and Gemology, Vol. 13, No. 4, Winter, pp. 120–122.
- Evans, B.D. (1994) Ubiquitous blue luminescence from undoped synthetic sapphire. Journal of Luminescence, Vol. 60–61, pp. 620–626.
- Hoover, D.B. and Theisen, A.F. (1993) Fluorescence excitation-emission spectra of chromium-containing gems: An explanation for the effectiveness of the crossed filter method. Australian Gemmologist, Vol. 18, No. 6, May, pp. 182–187.
- Hughes, R.W. (1997) Ruby & Sapphire. Boulder, CO, RWH Publishing, 512 pp.
- Robbins, M. (1994) Fluorescence: Gems and Minerals Under Ultraviolet Light. Phoenix, AZ, Geoscience Press, 374 pp.
- Wong, W.C., McClure, D.C. et al. (1995b) Charge-exchange processes in titanium-doped sapphire crystals. I. Charge-exchange energies and titanium-bound exitons. Physical Review B, Vol. 61, No. 9, pp. 5682–5692.
- Wong, W.C., McClure, D.C. et al. (1995a) Charge-exchange processes in titanium-doped sapphire crystals. II. Charge-transfer transition states, carrier trapping, and detrapping. Physical Review B, Vol. 61, No. 9, pp. 5693–5698.


