An orangish pink “padparadscha” sapphire was submitted for testing at Lotus Gemology’s Bangkok laboratory. Testing showed a number of conflicting features that suggested the gem was a cleverly treated synthetic pink sapphire designed to imitate natural padparadscha.
Introduction
In November of 2015, an orangish pink “padparadscha” sapphire of 14 ct was submitted for testing at Lotus Gemology’s Bangkok laboratory. At the time of take-in, the client stated that the gem’s origin was Sri Lanka, but the client was unsure of whether or not it had been treated.
Testing showed a number of conflicting features. Large fissures across the stone were filled with unusual orange stains displaying dendrite inclusions, along with needles along twin planes and what appeared to be tiny gas bubbles. Chemical analysis was performed by the Asian Institute of Gemological Sciences’ Bangkok lab. The lack of gallium and other features suggested this was a cleverly treated melt-grown synthetic pink sapphire designed to imitate padparadscha.
 Figure 1.The 14-ct sapphire that is the subject of this report. Photo: Wimon Manorotkul/Lotus Gemology
Figure 1.The 14-ct sapphire that is the subject of this report. Photo: Wimon Manorotkul/Lotus Gemology
 Figure 2. The same stone viewed from the back. One can clearly see the orange color in the fissure, with the pink color from the stone’s body. Photo: Wimon Manorotkul/Lotus Gemology. Click on the photo for a larger image.
Figure 2. The same stone viewed from the back. One can clearly see the orange color in the fissure, with the pink color from the stone’s body. Photo: Wimon Manorotkul/Lotus Gemology. Click on the photo for a larger image.
General Properties
Standard gemological testing revealed properties that were typical for corundum, with one exception. The typical ultraviolet fluorescence for padparadscha sapphires ranges from orange to red in both long wave (LW) and short wave (SW), with LW being stronger than SW. However this stone suspiciously showed SW fluorescence equal to that of LW.
Microscopic Features
Microscopic examination provided further clues to the stone’s identity. The most prominent internal feature was a large orange-stained fissure extending across half the stone (Figure 1). This added an orange color in the otherwise pink stone, superficially causing it to resemble a padparadscha sapphire.
The filling material was also quite unusual, featuring black dendritic inclusions and a glassy surface sheen at certain angles that resembled the filling of lead glass filled rubies and sapphires (Figures 3–5).
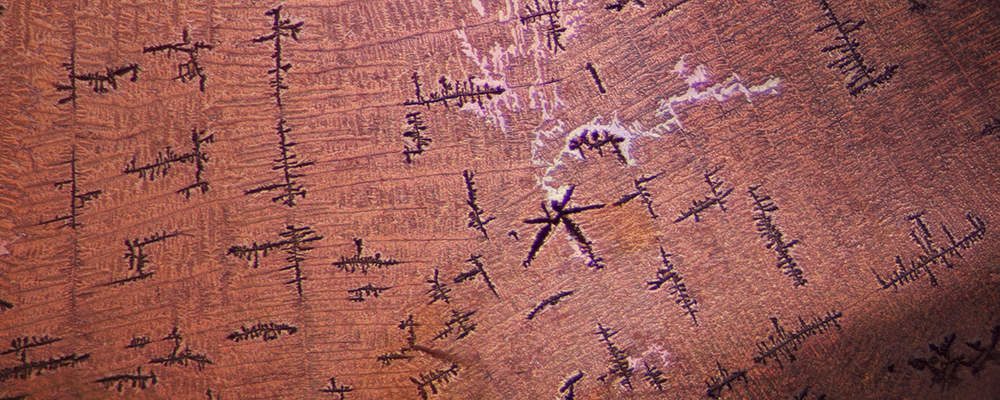
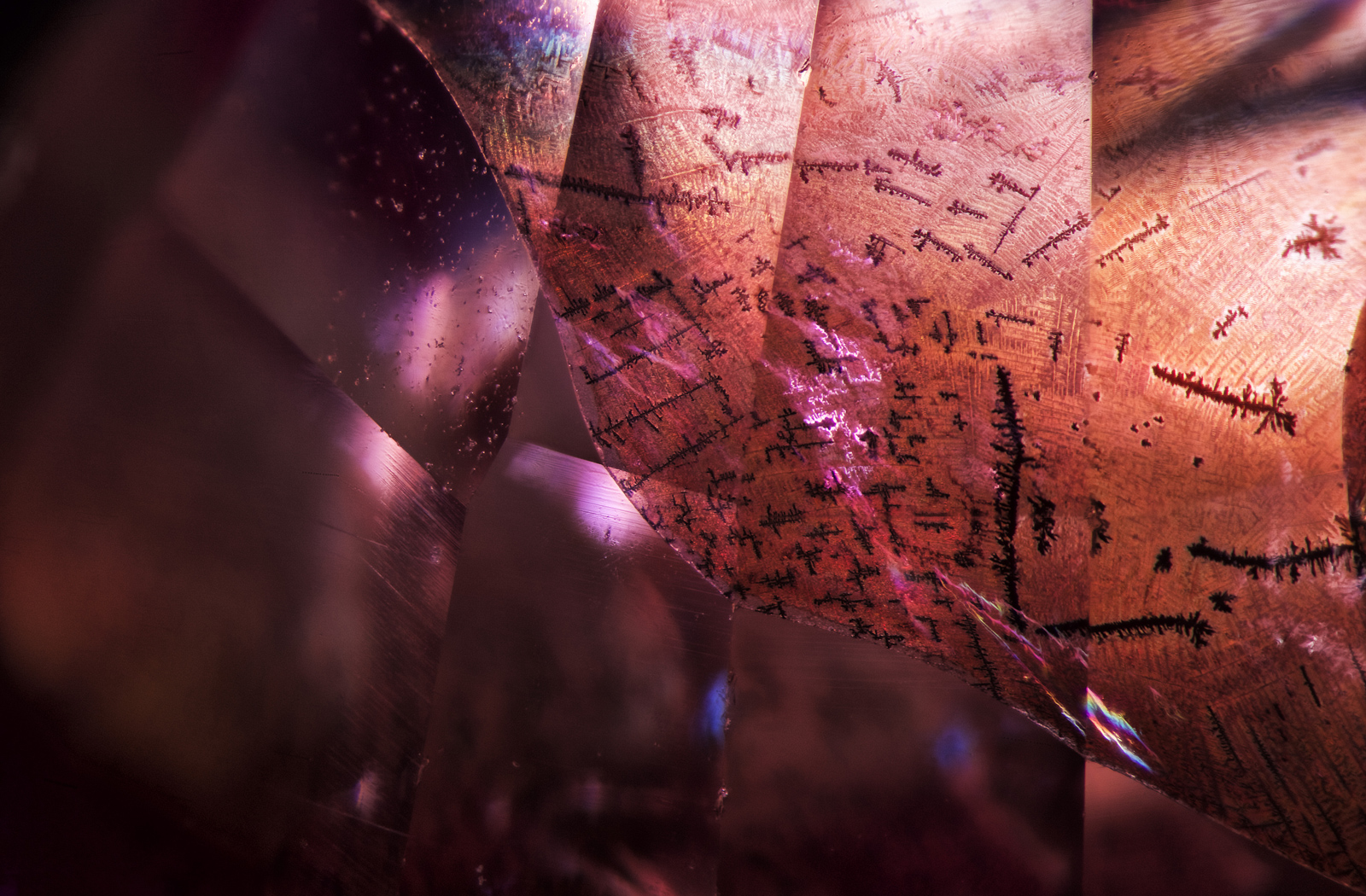 Figure 3. This large orange fissure was the major feature of an otherwise pink sapphire.
Figure 3. This large orange fissure was the major feature of an otherwise pink sapphire.
Photo: E. Billie Hughes/Lotus Gemology. Click on the photo for a larger image.
 Figure 4. Another view of the filled fissure with its black dendrite inclusions.
Figure 4. Another view of the filled fissure with its black dendrite inclusions.
Photo: Richard W. Hughes/Lotus Gemology. Click on the photo for a larger image.
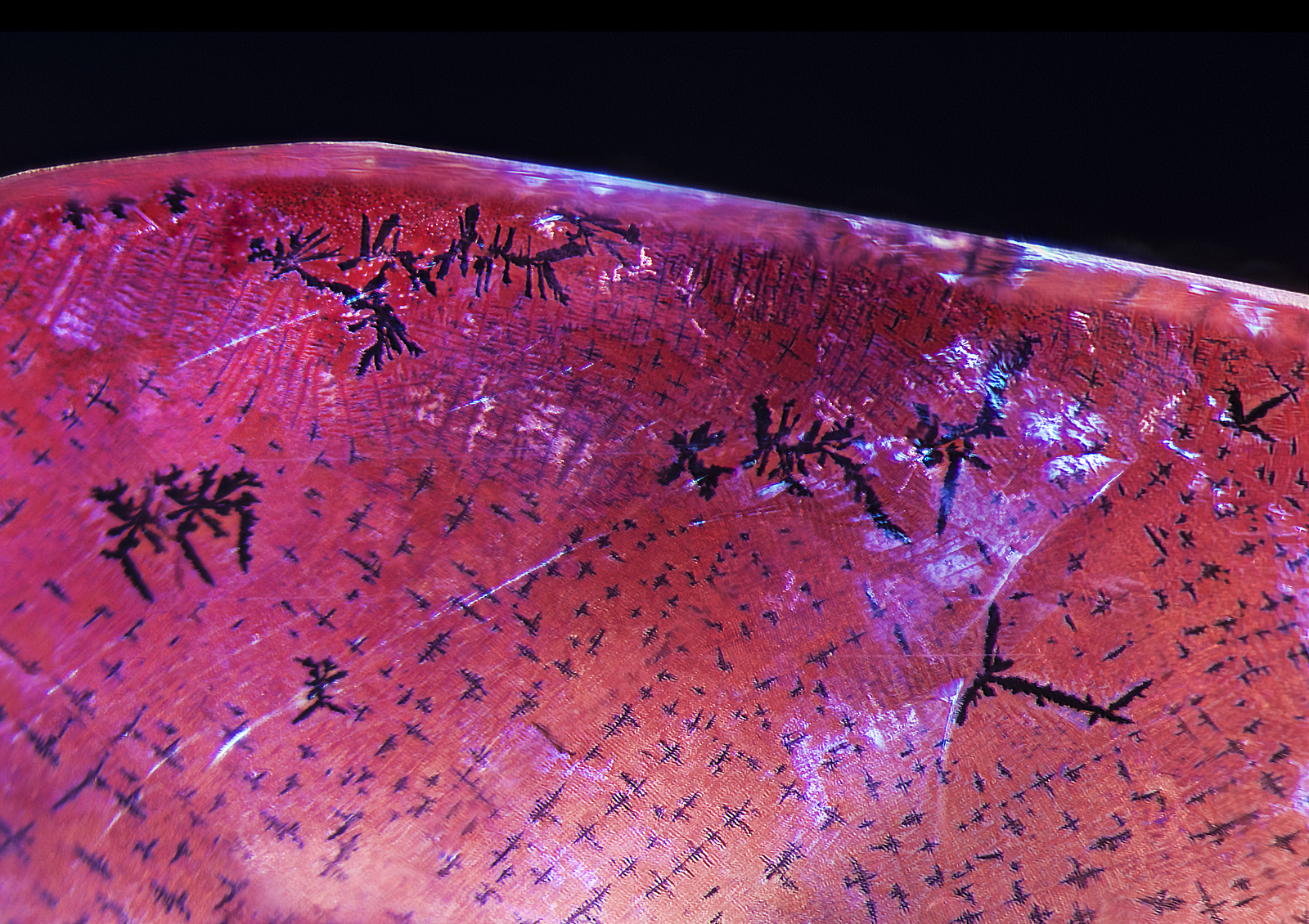 Figure 5. Under magnification, the large fissure displayed a number of unusual features, including black dendrites and a reflective sheen somewhat akin to that seen in glass-filled corundums.
Figure 5. Under magnification, the large fissure displayed a number of unusual features, including black dendrites and a reflective sheen somewhat akin to that seen in glass-filled corundums.
Photo: E. Billie Hughes/Lotus Gemology. Click on the photo for a larger image.
Further examination revealed dislocation needles along twin planes (Figure 6). While such needles are typical of natural corundum, on rare occasions they have been seen in synthetic corundums (Eppler, 1964; Hughes, 1997). Immersion between crossed polars revealed Plato-Sandmeier twinning (Figure 7).
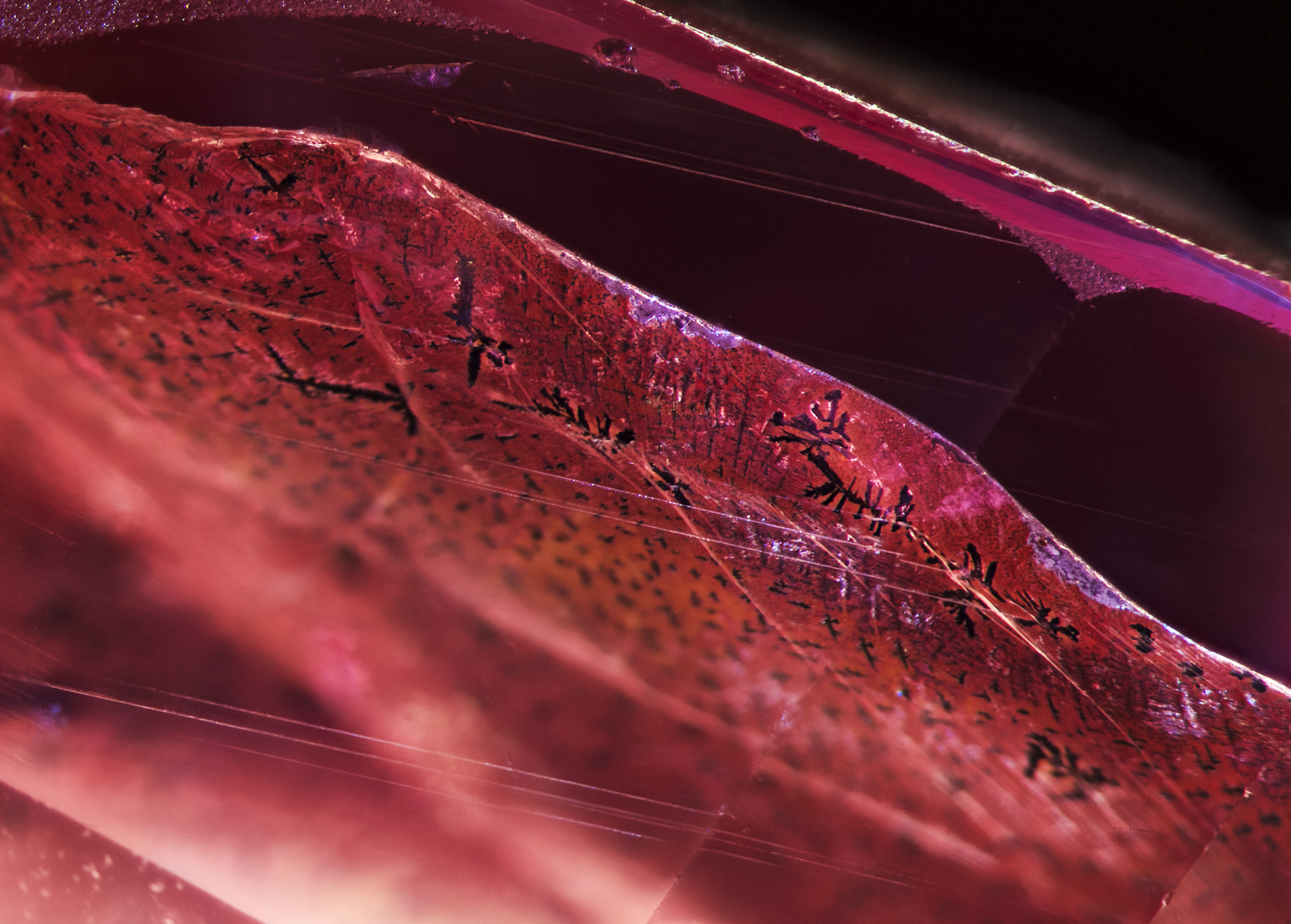 Figure 6. Parallel dislocation needles were seen in a single twin plane just above the fissure.
Figure 6. Parallel dislocation needles were seen in a single twin plane just above the fissure.
Photo: E. Billie Hughes/Lotus Gemology. Click on the photo for a larger image.
More disconcerting was the discovery of two tiny pinpoint inclusions in the pink portion of the stone. These were extremely small, but looked identical to the gas bubbles that are a common feature of melt-grown (Verneuil, Czochralski) synthetic corundums.
 Figure 7. When immersed in di-iodomethane (methylene iodide), the extent of the fissure becomes readily apparent. Adding polarized light reveals Plato-Sandmeier twinning. A dislocation needle can also be seen at the lower center of the stone. Photo: Richard W. Hughes/Lotus Gemology. Click on the photo for a larger image.
Figure 7. When immersed in di-iodomethane (methylene iodide), the extent of the fissure becomes readily apparent. Adding polarized light reveals Plato-Sandmeier twinning. A dislocation needle can also be seen at the lower center of the stone. Photo: Richard W. Hughes/Lotus Gemology. Click on the photo for a larger image.
Advanced Testing Methods
Non-destructive chemical analysis
The chemical analysis by ED-XRF (Energy-Dispersive X-Ray Fluorescence-Spectrometry) was performed using a Skyray Instrument EDX-6000B spectrometer with rotating table and spin option at AIGS in Bangkok. Full quantitative ED-XRF data with low error rates, high precision and good repeatability are achieved by calibration-curves using certified gem standards. Three different positions were analyzed, with the results given in Table 1.
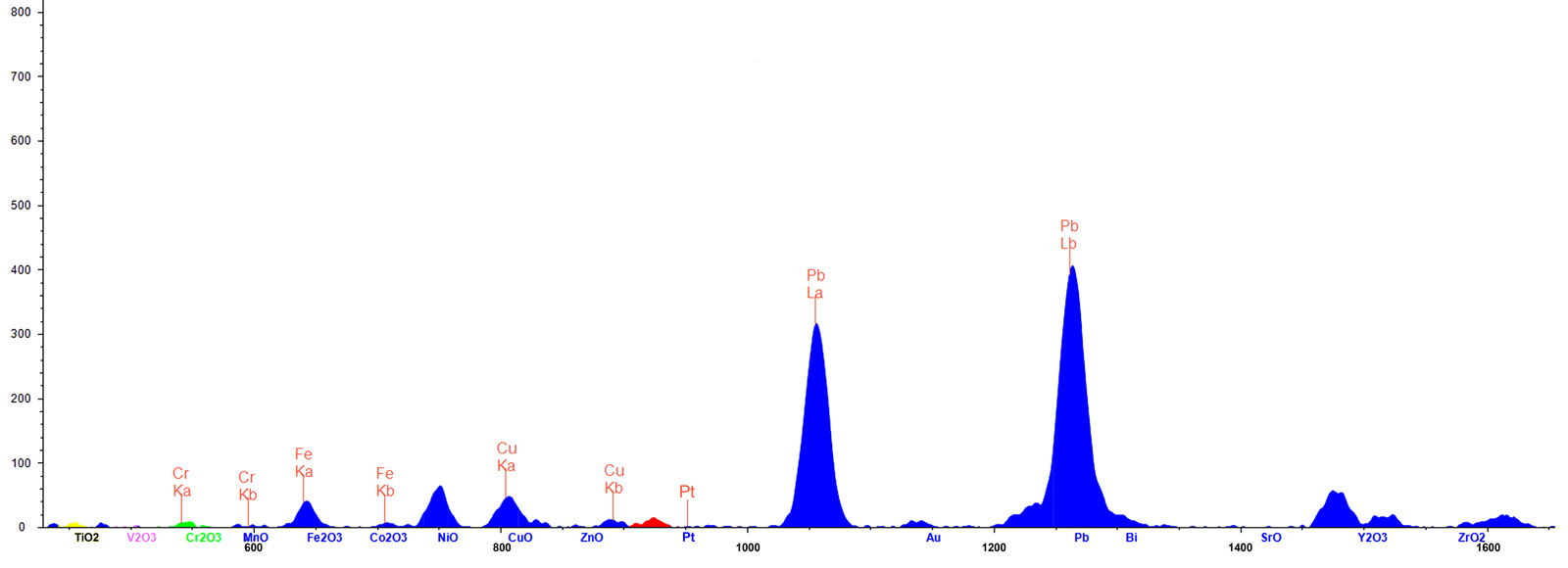 Figure 8. ED-XRF spectrum (45KV) of the fissure revealed unusually high levels of lead (Pb) and copper (Cu).Click on the photo for a larger image.
Figure 8. ED-XRF spectrum (45KV) of the fissure revealed unusually high levels of lead (Pb) and copper (Cu).Click on the photo for a larger image.
| Element | Position 1 (body) wt % | Position 2 (fissure) wt % | Position 3 (fissure) wt % |
|---|---|---|---|
| Al2O3 | 99.90 | 99.42 | 98.73 |
| Cl | 0.000 | 0.157 | 0.003 |
| K2O | 0.000 | 0.010 | 0.001 |
| CaO | 0.000 | 0.165 | 0.000 |
| TiO2 | 0.000 | 0.004 | 0.003 |
| V2O3 | 0.002 | 0.000 | 0.000 |
| Cr2O3 | 0.037 | 0.035 | 0.041 |
| Fe2O3 | 0.000 | 0.008 | 0.001 |
| CuO | 0.000 | 0.006 | 0.020 |
| Pb | 0.001 | 0.165 | 1.027 |
| Na2O, MgO, SiO2 | 0.00 | 0.00 | 0.00 |
| Sc2O3, CoO, NiO, ZnO Ga2O3, W, Ir, Pt, Au |
0.000 | 0.000 | 0.000 |
Detection limits: Atomic Numbers 11–14 = 100 ppm, Atomic Numbers15–92 = 10 ppm (Table 1).
Two elements are significant in the corundum body, as follows:
- Gallium (Ga2O3), which is typically found in all natural corundum, is completely absent; this suggests the stone is of synthetic origin.
- Chromium shows up in concentrations from 340 to 410 ppm (0.035 to 0.041% Cr2O3). This produces the pink color.
Two elements of significance were also found in the fissure, as follows:
- While lead was found in tiny amounts (10 ppm; 0.001% Pb) in the non-fracture area, high concentrations of more than 10,000 ppm (1.027% Pb) were seen in the fissure, suggesting it had been filled with lead glass (see Figure 7). Pb-rich glass is commonly used for clarity enhancement of rubies and sapphires (McClure & Smith et al., 2006; Choudhary, 2014; Leelawatanasuk & Susawee et al., 2015).
- Surprisingly, copper was also found in the fissure. We suspect the copper unmixed to form the black dendrites. Artificially created dendrites have also been described in the literature (Fischer, 1991; Johnson & Koivula et al., 2000).
Tungsten (W), platinum (Pt) and gold (Au) are automatically analyzed in corundum measurement routines as well, as they might indicate a synthetic stone made by a crucible growth production (flux, hydrothermal). In this stone, no traces of these metals were detected. Iridium, which is commonly used as a crucible container in the Czochralski process, was also not detected. The presence of low concentrations of chlorine (Cl), potassium (K) and calcium (Ca) in the fissure appear to be components of the glass filler.
According to Muhlmeister & Fritsch et al.’s groundbreaking article on trace element analysis of ruby (Muhlmeister & Fritsch et al., 1998), “…the presence of Mo, La, W, Pt, Pb or Bi proves that a ruby is synthetic. Ni and Cu suggest synthetic origin…”. While they add the caveat that Ni and Cu could be present in sulfide inclusions in natural rubies, their research strongly suggests that the presence of Pb and Cu by themselves build a strong case for synthetic origin. Adding to this the complete absence of Ga and the conclusion is inescapable: this stone is of synthetic origin.
Infrared spectroscopy
The sample’s mid-infrared spectra (Figure 9), showed a series of bands at 2854 cm–1, 2923 cm–1 and 2958 cm–1. These bands are due to oil or grease from human fingerprints. The fuzzy and complex bands in the 3500-4000 cm–1 region, and the sharp peak at 2354 cm–1 resulted from the atmospheric fluctuations such as moisture and CO2 during the measurement. However, there was an absence of absorption bands related to the hydroxyl group or any hydrous minerals as previously reported in both natural and synthetic sapphires (Volynets & Vorob’ev et al., 1969; Moon and Phillips, 1994; Smith, 1995; Balmer & Leelawatanasuk et al., 2006; Beran & Rossman, 2006; Smith & van der Bogert, 2006).
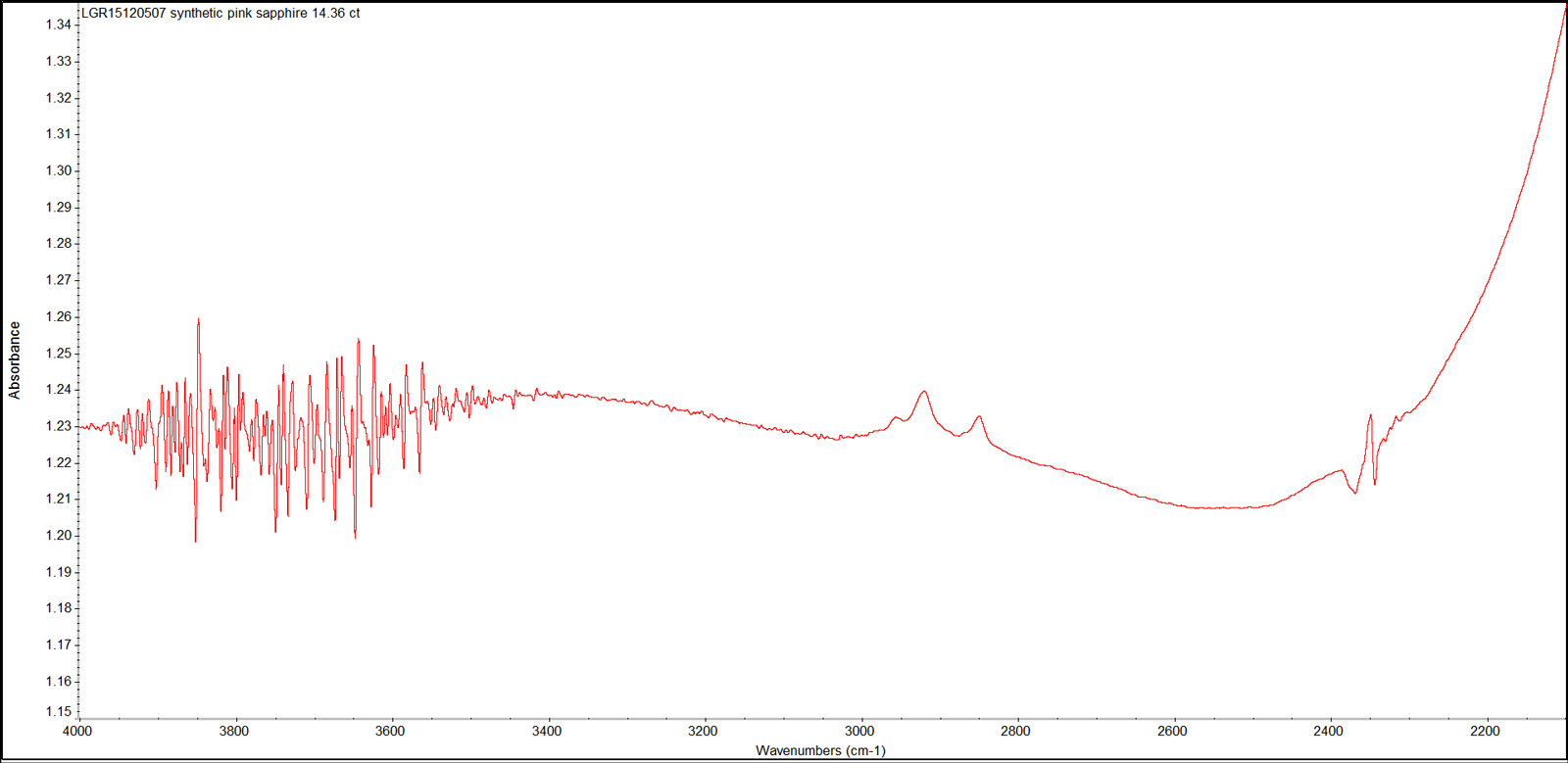 Figure 9. This representative infrared absorption spectrum displays the series of bands due to oil or grease contamination (2800–3000 cm–1), and the atmospheric CO2 band at 2354 cm–1. FTIR spectrum was recorded in absorbance mode using a Thermo Nicolet iS50 FTIR spectrometer in the range 4000–400 cm–1, with a resolution of 4.0 cm–1 and 20 scans. Spectrum: Chawalit Chankhantha/AIGS. Click on the photo for a larger image.
Figure 9. This representative infrared absorption spectrum displays the series of bands due to oil or grease contamination (2800–3000 cm–1), and the atmospheric CO2 band at 2354 cm–1. FTIR spectrum was recorded in absorbance mode using a Thermo Nicolet iS50 FTIR spectrometer in the range 4000–400 cm–1, with a resolution of 4.0 cm–1 and 20 scans. Spectrum: Chawalit Chankhantha/AIGS. Click on the photo for a larger image.
Conclusions
Decades of testing precious stones in the lab have driven home many key lessons. But perhaps none is more important than that of the need to exercise great caution when confronted with a gem that presents contradictory evidence.
The late, great B.W. Anderson once wrote that the most common testing mistake by gemologists was a failure to consider enough possibilities. This was certainly true with this “padparadscha.” A hurried look would reveal the orange stains and dendrites in the fissure, and the dislocation needles and twinning would reinforce the view that this was a natural stone. Indeed, some of the junior gemologists who examined it first believed it to be genuine.
But the patient gemologist would reserve judgment, taking note of the unusual UV fluorescence (why is SW equal to LW?), the two tiny pinpoint inclusions (gas bubbles or crystals?) and the twinning (natural or Plato-Sandmeier type?).
Further testing would reveal trace-element chemistry in the pink portion of the stone that lacked gallium. In contrast, significant quantities of Pb and Cu were found in the fissure, with the Cu possibly unmixing as dendrites.
The result of the full collection of data is unmistakable—a “padparadscha” unmasked as a cleverly crafted pretender—a Verneuil melt-grown synthetic pink sapphire that had been artificially fractured and then had the fissure filled with a Pb-rich glass with traces of Cu.

About the authors
E. Billie Hughes visited her first gem mine (in Thailand) at age two and by age four had visited three major sapphire localities in Montana. A 2011 graduate of UCLA, she qualified as a Fellow of the Gemmological Association of Great Britain (FGA) in 2013. An award winning photographer and photomicrographer, she has won prizes in the Nikon Small World and Gem-A competitions, among others. Her writing and images have been featured in books, magazines, and online by Forbes, Vogue, National Geographic, and more. In 2019 the Accredited Gemologists Association awarded her their Gemological Research Grant. Billie is a sought-after lecturer and has spoken around the world to groups including Cartier and Van Cleef & Arpels. In 2020 Van Cleef & Arpels’ L’École School of Jewellery Arts staged exhibitions of her photomicrographs in Paris and Hong Kong.
Chawalit Chankhantha was at the time of publication working in the Bangkok lab of the Asian Institute of Gemological Sciences.
Dr. Andreas Burkhardt is one of the world's foremost authorities on ED-XRF and the President of Xray Analytics in Zürich, Switzerland.
Wimon Manorotkul has been involved with gems and gemology since 1979, as a lab gemologist, instructor and photographer. She is an Accredited Gemologist from Bangkok's Asian Institute of Gemological Sciences and for many years directed their lab. Wimon also qualified as a Fellow (with honors) of the Gemmological Association of Great Britain. A skilled gem photographer, her images have been featured in books and magazines around the world, particularly Ruby & Sapphire: A Collector's Guide, along with Ruby & Sapphire: A Gemologist's Guide and Inside Out | GEM• ology Through Lotus-Colored Glasses. Wimon not only photographs gems, jewelry and mineral specimens, but is also an expert photomicrographer. In 2013, she founded Lotus Gemology with her husband, Richard Hughes, and daughter, E. Billie Hughes.
Richard W. Hughes is one of the world’s foremost experts on ruby and sapphire. The author of many books and over 170 articles, his writings and photographs have appeared in a diverse range of publications, and he has received numerous industry awards. Co-winner of the 2004 Edward J. Gübelin Most Valuable Article Award from Gems & Gemology magazine, the following year he was awarded a Richard T. Liddicoat Journalism Award from the American Gem Society. In 2010, he received the Antonio C. Bonanno Award for Excellence in Gemology from the Accredited Gemologists Association. The Association Française de Gemmologie (AFG) in 2013 named Richard as one of the Fifty most important figures that have shaped the history of gems since antiquity. In 2016, Richard was awarded a visiting professorship at Shanghai's Tongji University. 2017 saw the publication of Richard and his wife and daughter's Ruby & Sapphire • A Gemologist's Guide, arguably the most complete book ever published on a single gem species and the culmination of four decades of work in gemology. In 2018, Richard was named Photographer of the Year by the Gem-A, recognizing his photo of a jade-trading market in China, while in 2020, he was elected to the board of directors of the Accredited Gemologists Association and was appointed to the editorial review board of Gems & Gemology and The Australian Gemmologist magazine. In 2022, Richard published Jade • A Gemologist's Guide, while 2024 brought Broken Bangle • The Blunder-Besmirched History of Jade Nomenclature. His jade trilogy was completed in 2025 with his translation of Heinrich Fischer's Nephrite and Jadeite.
Notes
First published in the Australian Gemmologist (2016, Vol. 25, Nos. 11–12, pp. 389–392).
References & further reading
- Balmer, W.A., Leelawatanasuk, T., Atichat, W., Wathanakul, P., and Somboon, C. (2006) Update on characteristics of heated yellow sapphires. Poster, Proceedings of the 1st International Gem and Jewelry Conference, 3 pp.
- Beran, A., and Rossman, G.R. (2006) OH in naturally occurring corundum. European Journal of Mineralogy, Vol. 18, No. 4, pp. 441–447.
- Choudhary, G. (2014) Gem News International: Yellow sapphire filled with lead glass. Gem & Gemology, Vol. 50, No. 1, Spring, pp. 92–93.
- Eppler, W.F. (1964) Polysynthetic twinning in synthetic corundum. Gems & Gemology, Vol. 11, No. 6, Summer, pp. 169–175.
- Fischer, G.W. (1991) Gemstone & Chemicals: How to Create Color and Inclusions. No city, self published, 74 pp.
- Hughes, R.W. (1997) Ruby & Sapphire. Boulder, CO, RWH Publishing, 512 pp.
- Johnson, M.L., Koivua, J.I. et al. (2000) Gem News International: Unusual treated chalcedony. Gems & Gemology, Vol. 36, No. 2, Summer, pp. 170–171.
- Leelawatanasuk, T., Susawee, N. et al. (2015) Green lead-glass-filled sapphires. Journal of Gemmology, Vol. 34, No. 5, pp. 420–427.
- McClure, S.F., Smith, C.P. et al. (2006) Identification and durability of lead glass-filled rubies. Gems & Gemology, Vol. 42, No. 1, Spring, pp. 22–34.
- Moon, A.R. and Phillips, M.R. (1994) Defect clustering and colour in Fe, Ti: αAl2O3. Journal of the American Ceramic Society, Vol. 77, No. 2, pp. 356–367.
- Muhlmeister, S., Fritsch, E. et al. (1998) Separating natural and synthetic rubies on the basis of trace-element chemistry. Gems & Gemology, Vol. 34, No. 2, Summer, pp. 80–101.
- Schmetzer, K. and Schwarz, D. (2004) The causes of colour in untreated, heat treated and diffusion treated orange and pinkish orange sapphires – a review. Journal of Gemmology, Vol. 29, No. 3, July, pp. 149–181.
- Smith, C.P. (1995) A contribution to understanding the infrared spectra of rubies from Mong Hsu, Myanmar. Journal of Gemmology, Vol. 24, No. 5, Jan., pp. 321–335.
- Smith, C.P. and van der Bogert, C. (2006) Infrared spectra of gem corundum. Gems & Gemology, Vol. 42, No. 3, Fall, pp. 92–93.
- Volynets, F.K., Vorob’ev, V.G. et al. (1969) Infrared absorption bands in corundum crystals. Journal of Applied Spectroscopy, Vol. 10, No. 6, pp. 665–667.