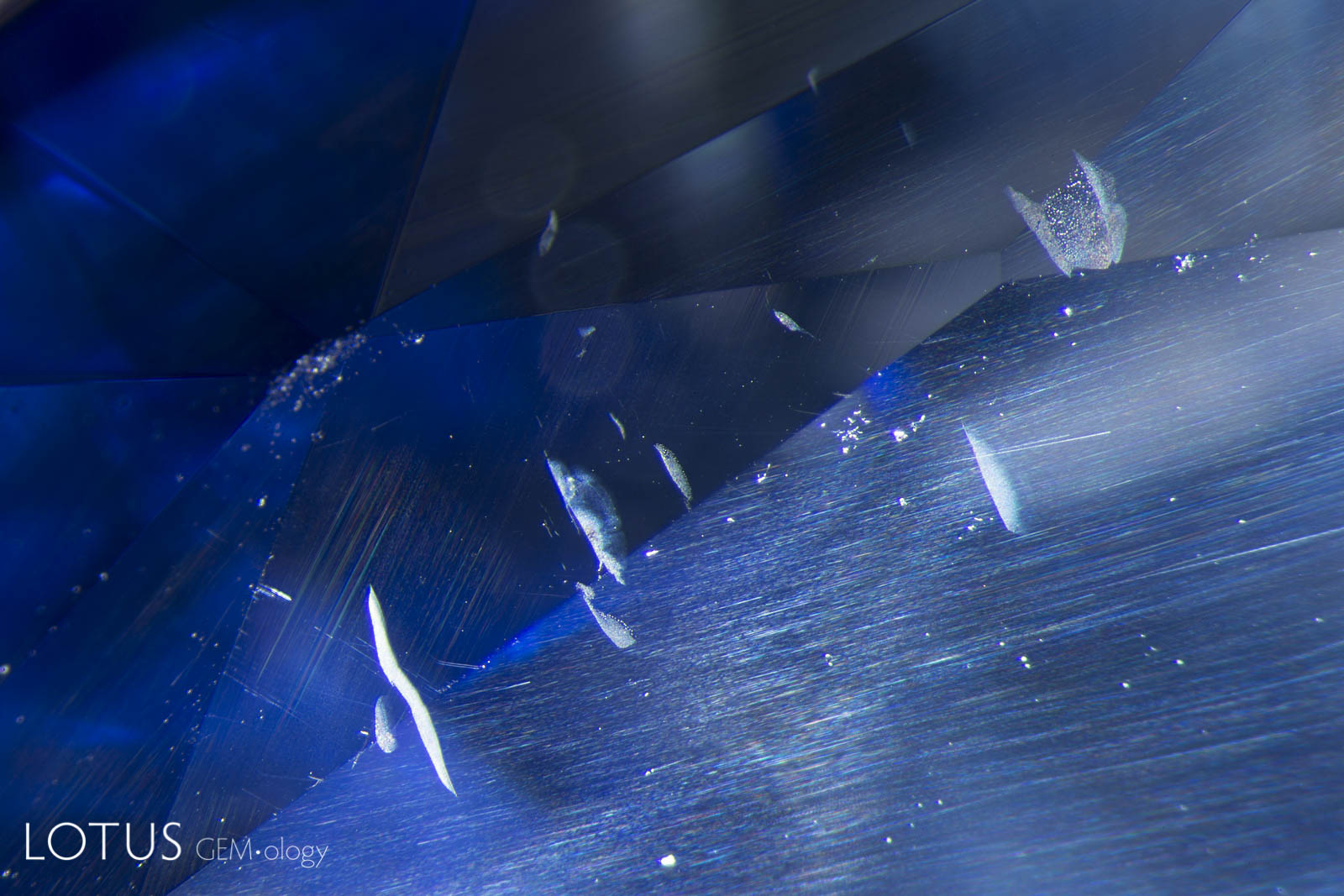
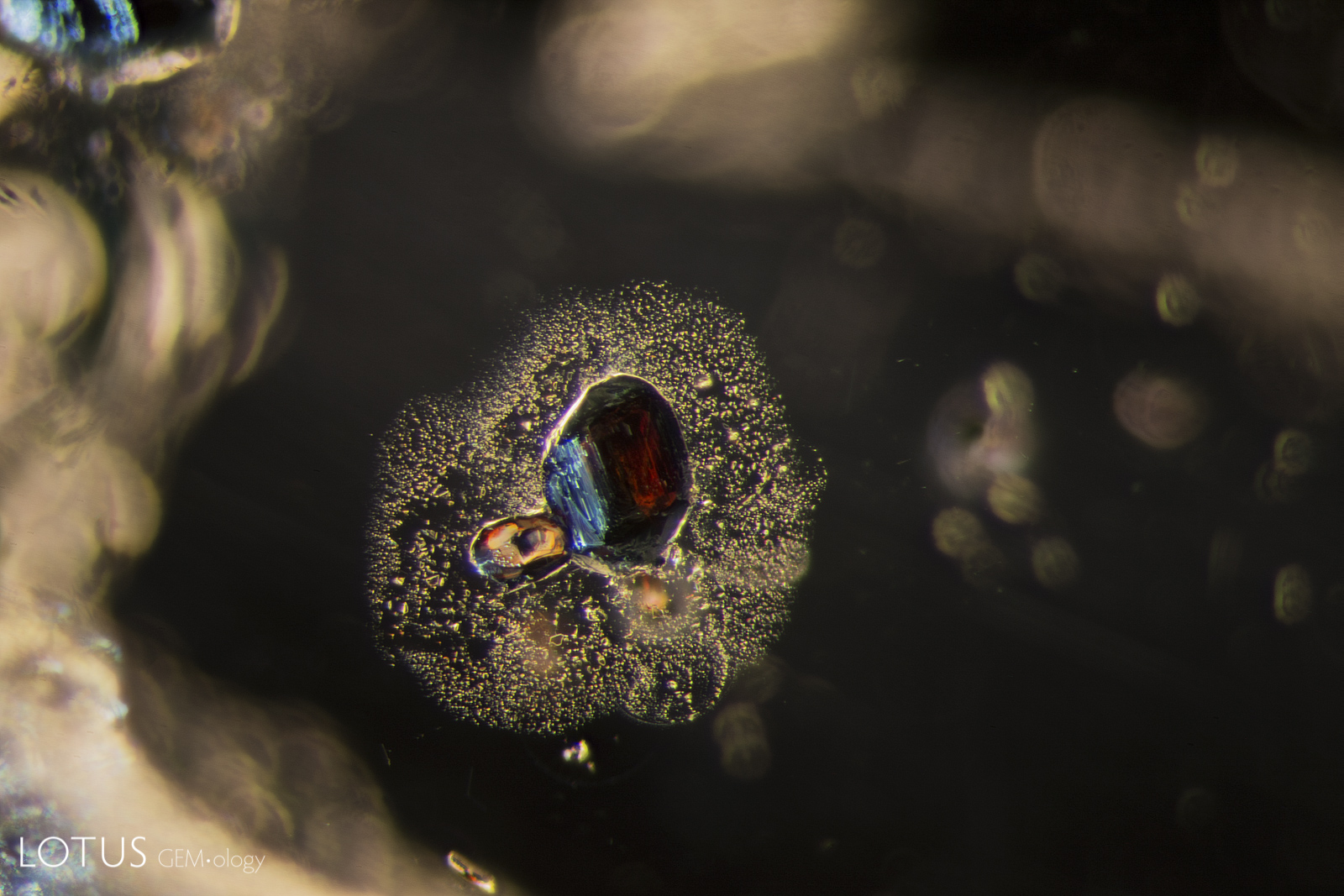
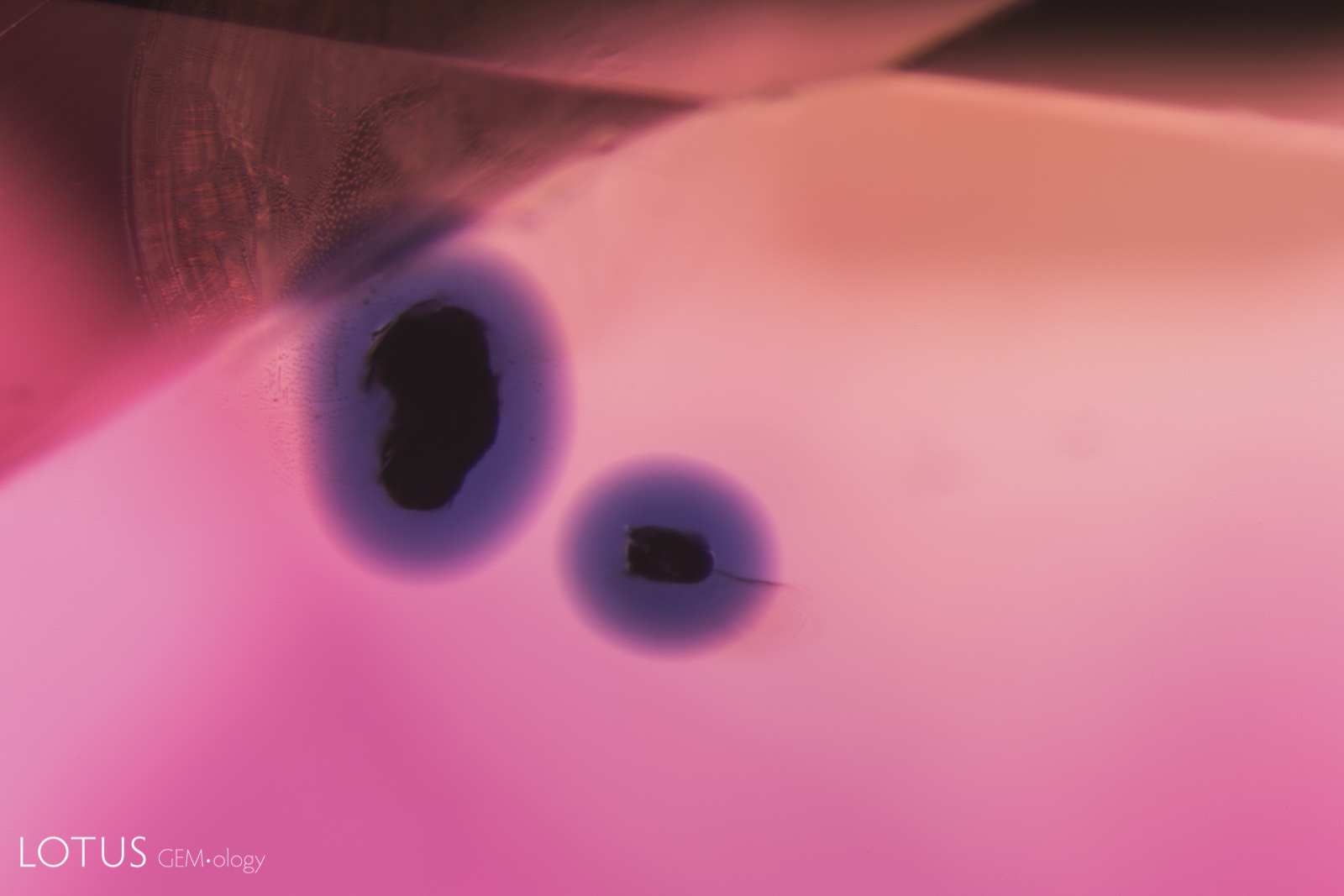
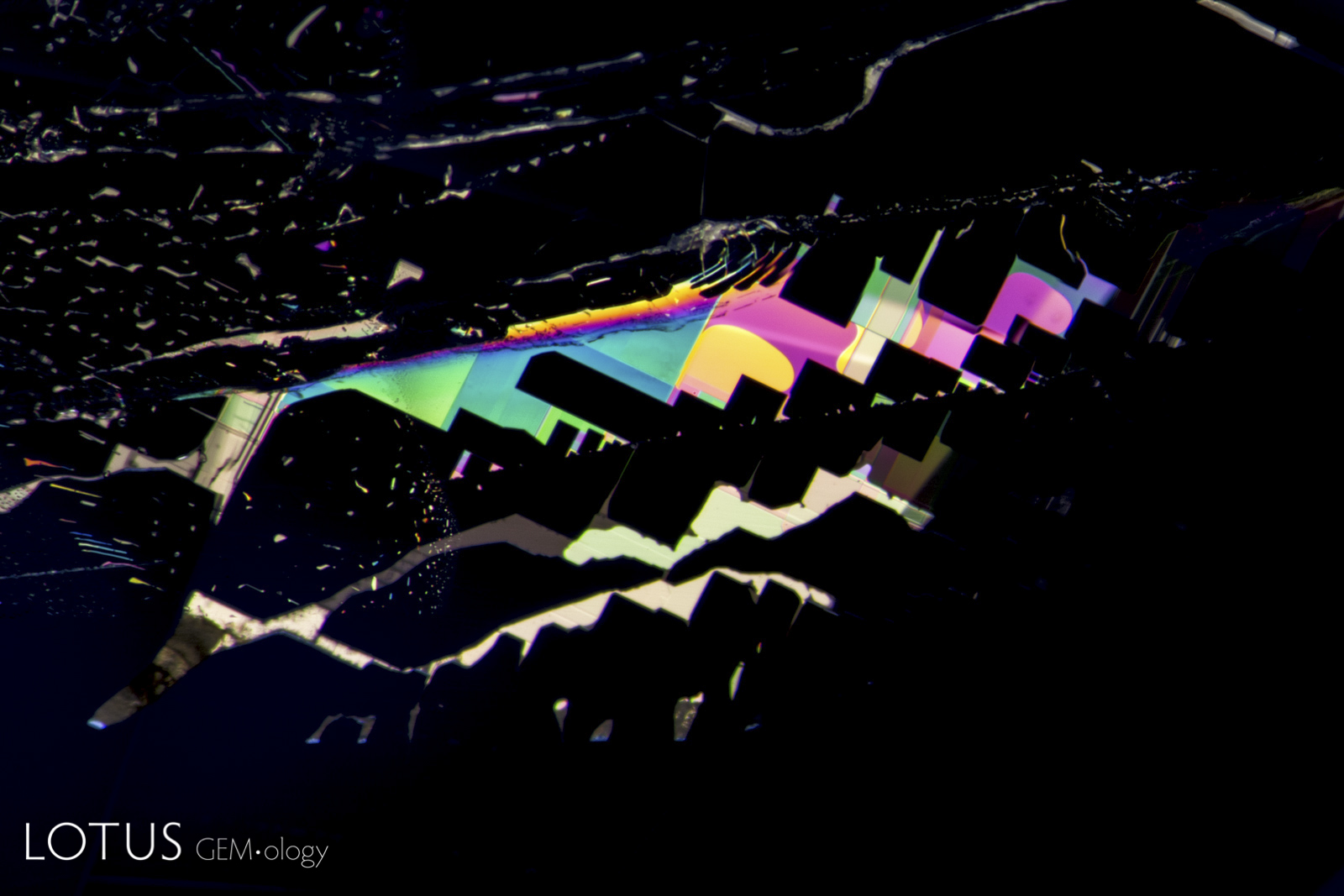
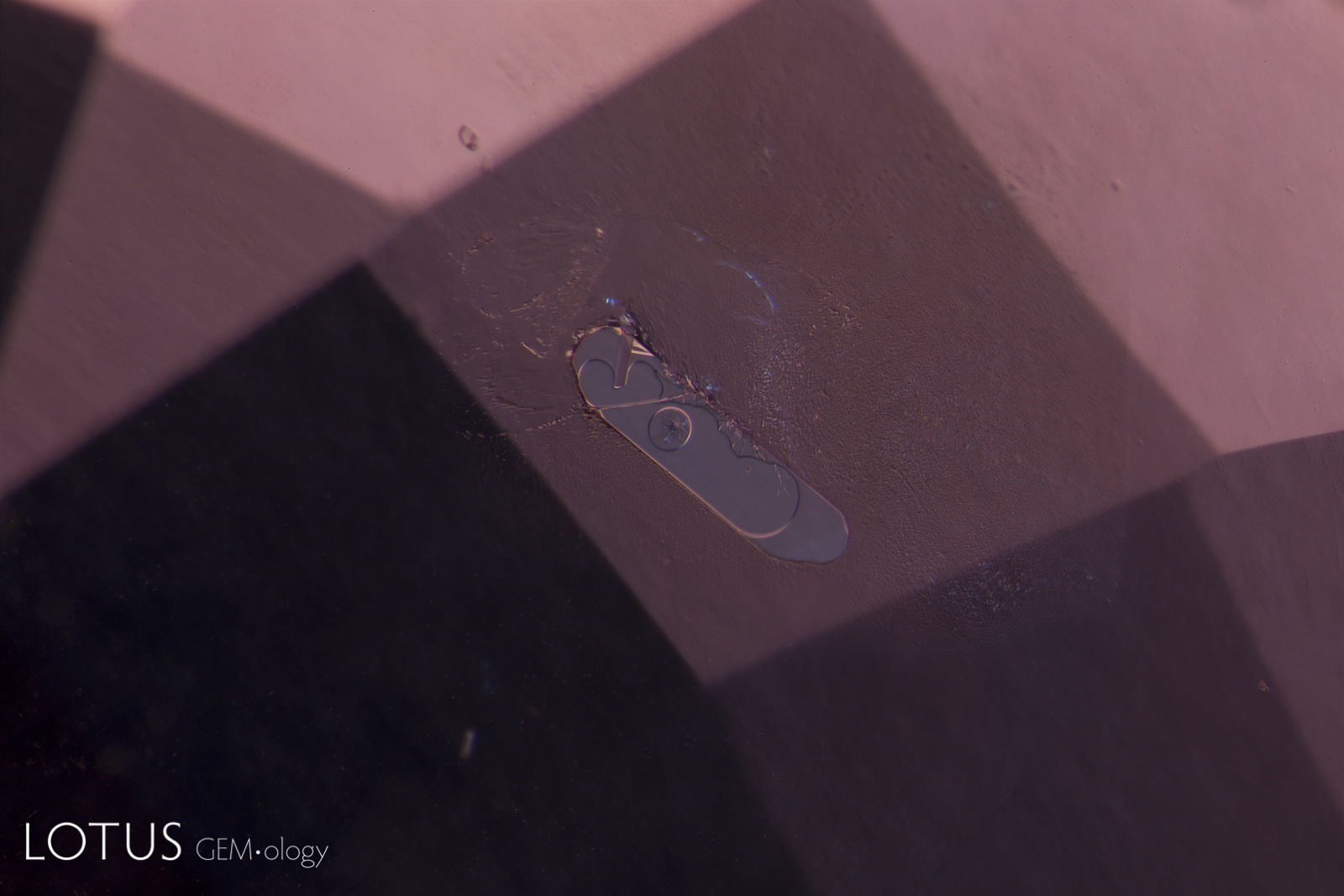This special gallery features paired images from the Lotus Gemology Hyperion Inclusion Database, along with other pairs of spectra, etc., allowing one to directly compare contrasting features.
Same same… but different.
Common Thai Saying
Showing 1 – 20 of 71 sets.
Scroll down to browse the sets or filter the results with the search box above.
Click on any photo for a larger image.
A. In contrast to B-jade, the surface of this high-quality untreated fei cui jade from Myanmar shows virtually no fissures and slight undercutting from one mineral grain to the next (fei cui is a rock composed of many small crystals). Specimen courtesy of Kiarttichatra Intarungsee.
B. The first step of preparing a piece of fei cui jade for polymer impregnation is to boil it in acid to remove any mineral impurities from the tiny fissures between the individual crystal grains that make up the jade rock. These fissures are then filled with a polymer. Thus the treatment removes brown discoloration and greatly improves the translucency. However the telltale micro-fissures from the bleaching process can still be seen by examining the surface with overhead light, as shown here. Polymer-impregnated jade is termed “B-jade” in the trade. Untreated jade is known as “A-jade,” while dyed jade is “C-jade.” If a dyed polymer is used, it is termed “B+C jade.”
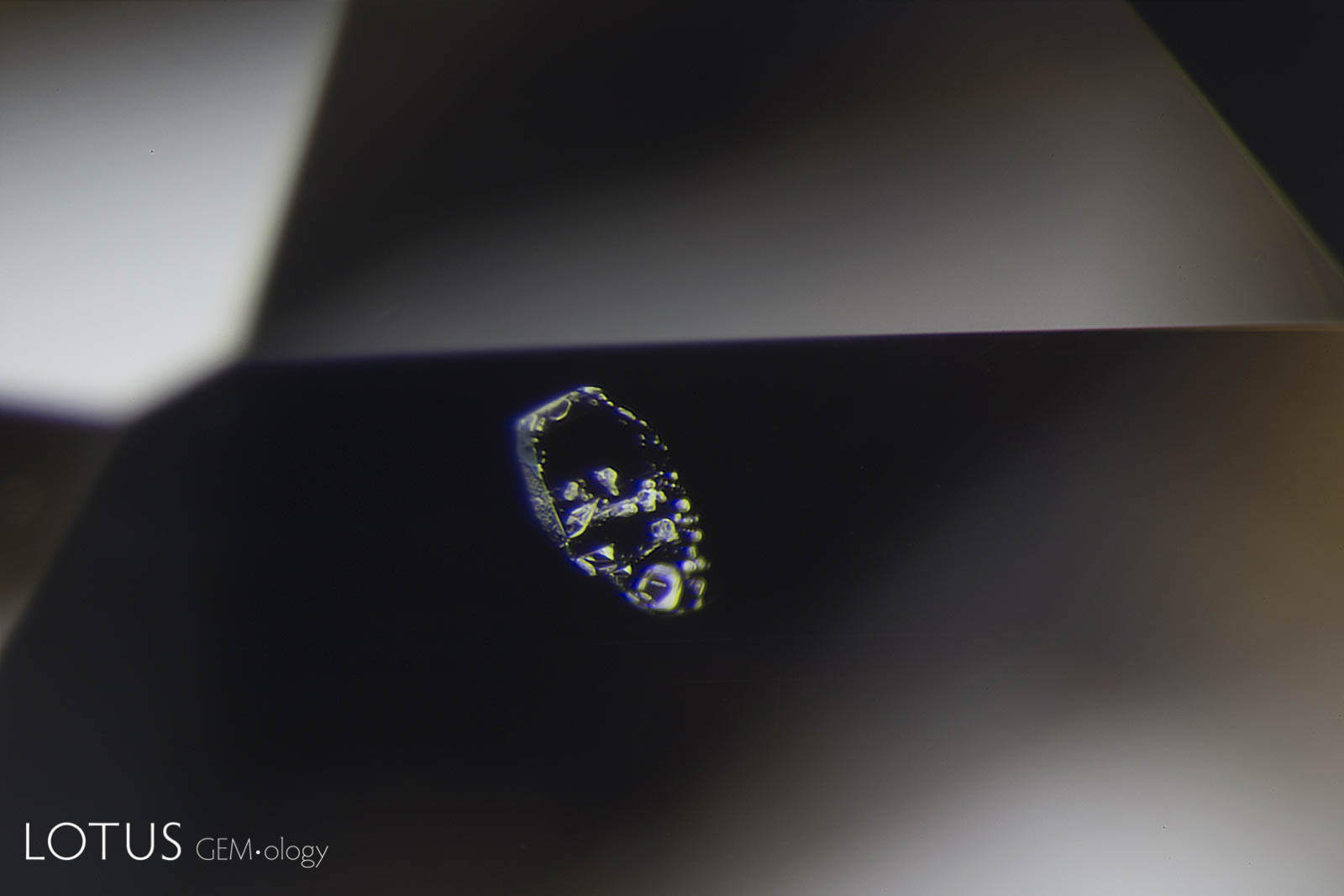
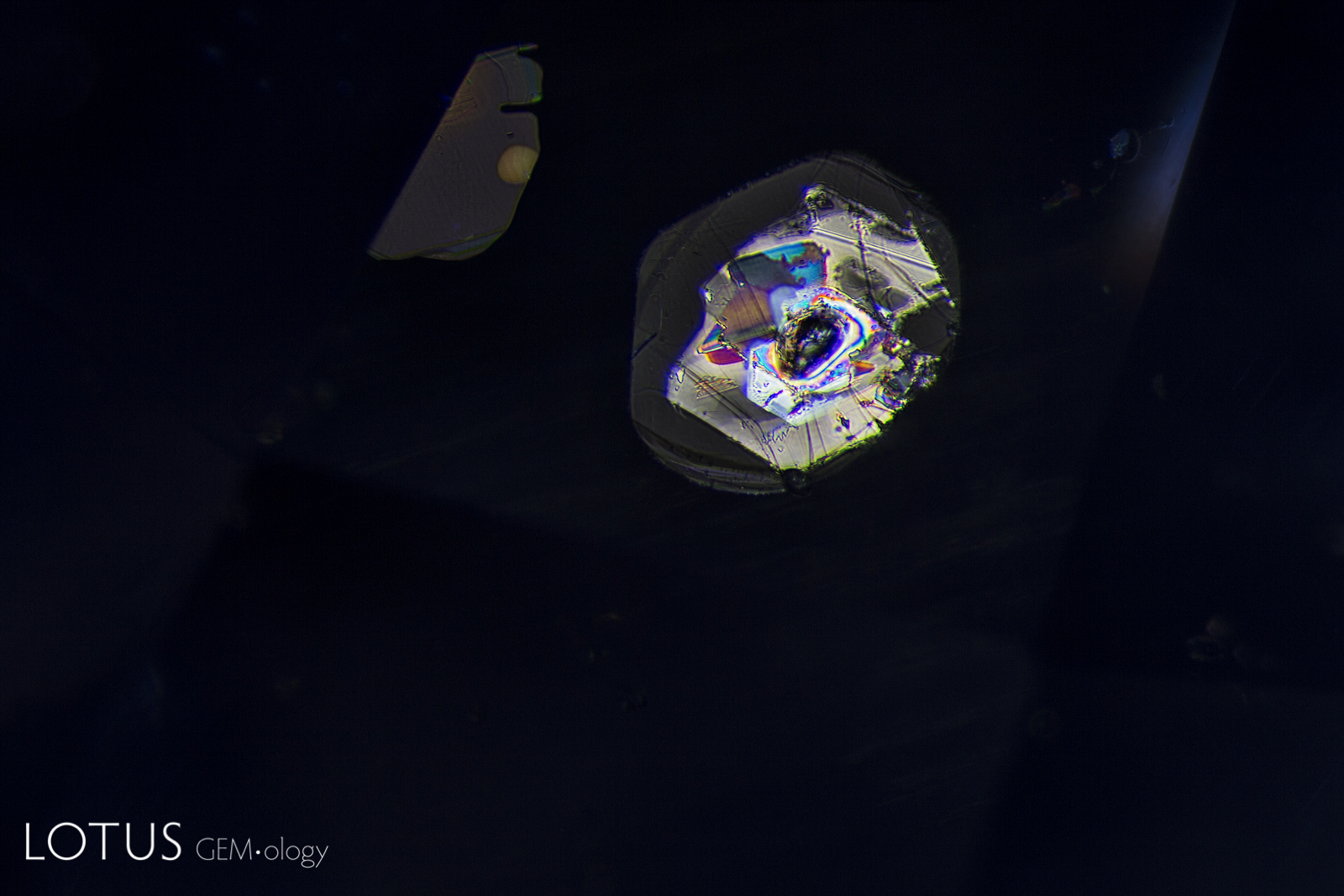
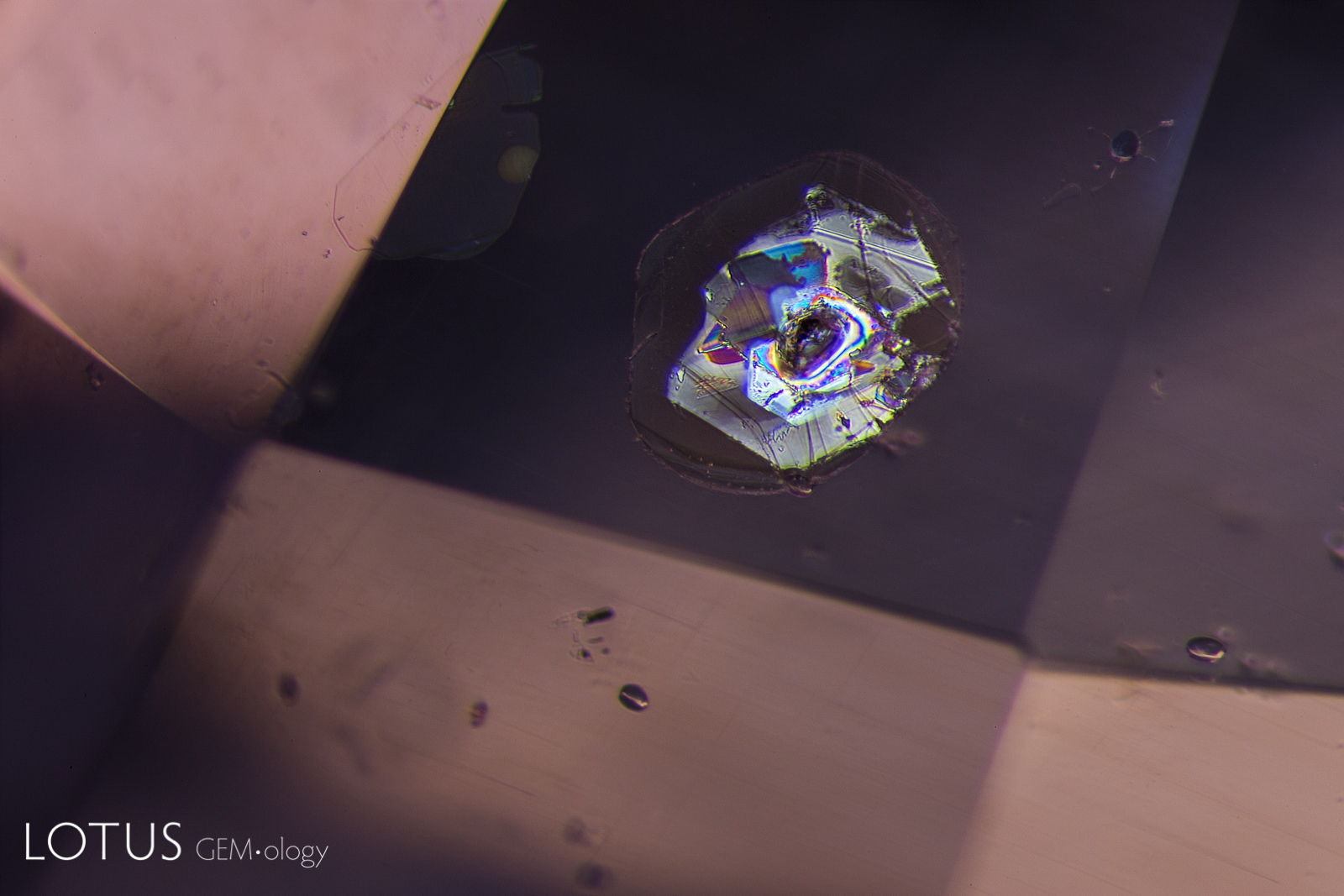
A. These included hexagonal crystals in a Sri Lankan blue sapphire have inclusions of their own. These are actually sapphire crystals captured in a sapphire host, as shown by their extremely low relief. Because of the low relief, the outlines of the crystals are difficult to discern.
B. In darkfield (left) they are hard to distinguish from the surrounding corundum, but in crossed polars (right) it’s apparent that they are distinct crystals of their own.
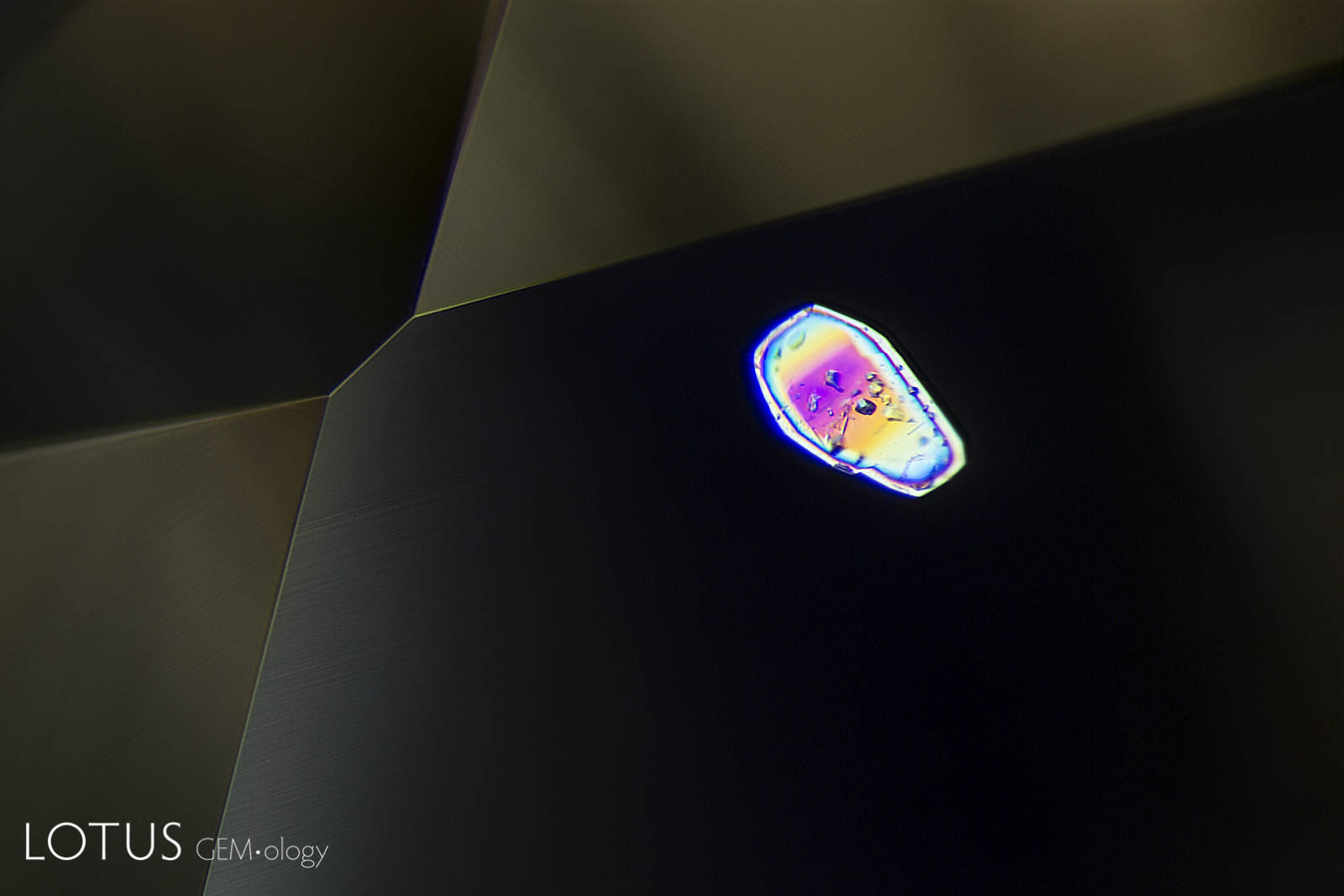
A. Another example of sapphire crystal included in a Ceylon sapphire, this time a yellow sapphire. In darkfield the outline of the sapphire crystal is hard to discern.
B. Switching to crossed polars illuminates the sapphire inclusion dramatically.
A. When viewed in transmitted light, this negative crystal within an untreated Sri Lanka padparadscha displays a hexagonal plate of graphite, along with a needle of diaspore. The undamaged nature of the negative crystal and diaspore needle confirms that the gem has not been heat treated.
B. Using overhead reflected light on the same negative crystal, one can see the high-relief of the hexagonal plate of graphite. The diaspore needle has a refractive index quite close to the surrounding corundum, and so "disappears" into the corundum matrix. The undamaged nature of the negative crystal and diaspore needle confirms that the gem has not been heat treated.
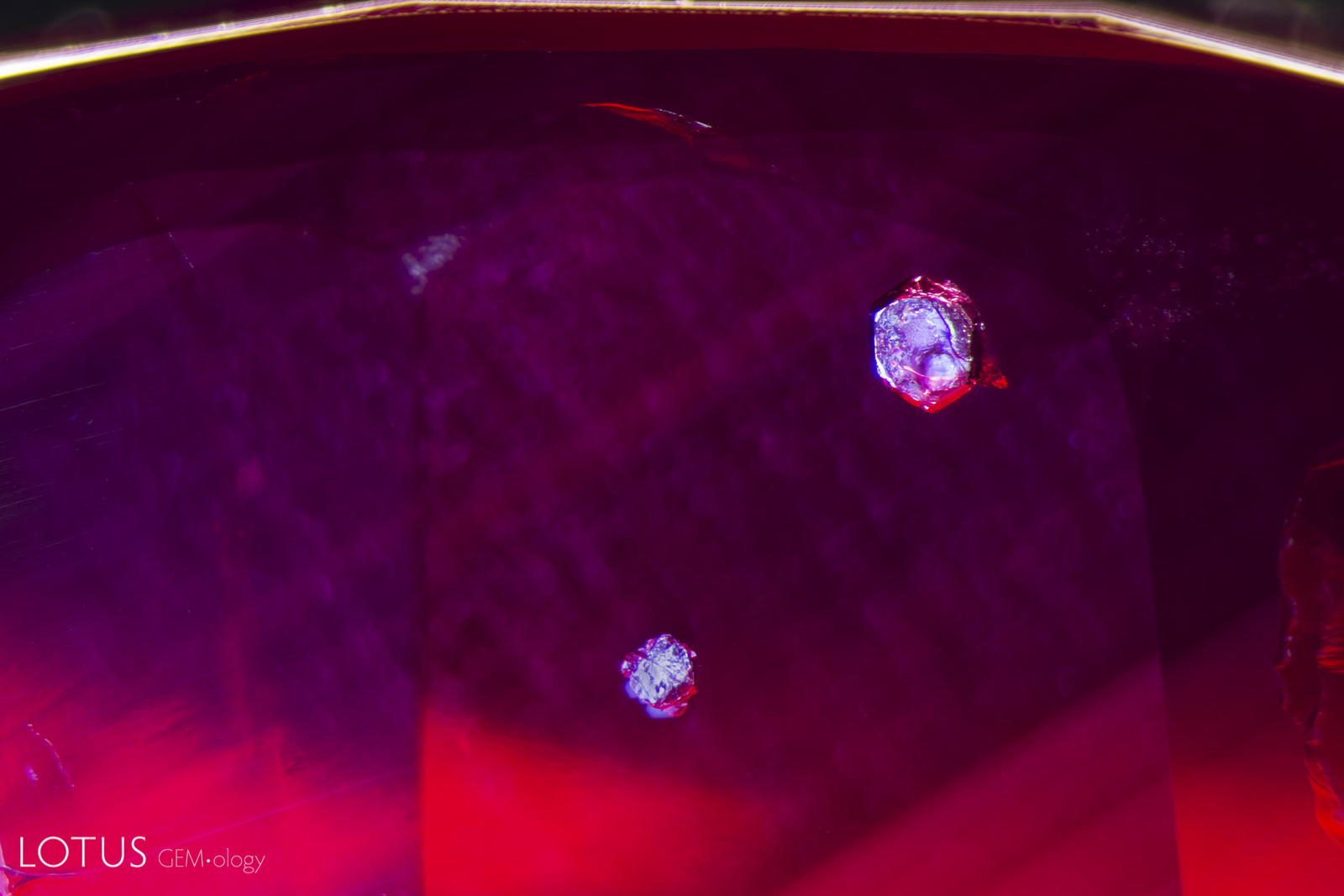
A. This red spinel from Tanzania’s Mahenge mines showed an unusual orange coloration surrounding a fingerprint inclusion.
B. When examined in oblique fiber-optic lighting, the orange area displays milky bluish clouds. This unmasks the orange color as a function of scattering, the same phenomena that creates a blue sky and orange sunset.
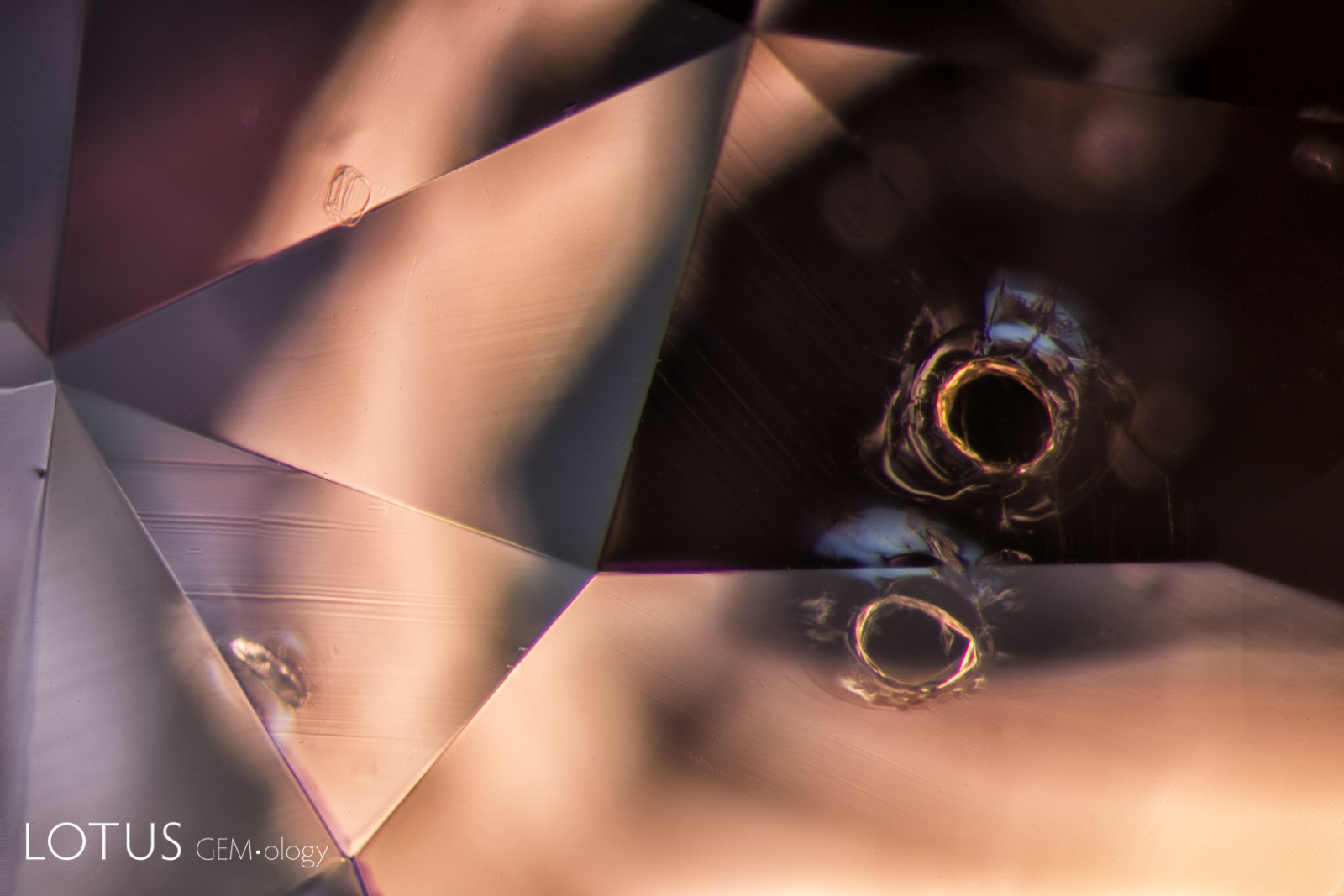
A. In this heated and fissure-healed ruby from Myanmar, a surface cavity is filled with glass. The glass can be identified by the gas bubble at the lower part of the cavity.
B. The same cavity when viewed with diffuse overhead illumination, which clearly reveals its extent. The glass can be identified by its lower luster.
A. Demonstrating that the inner world can be as captivating as that outside, this Madagascar sapphire displays a small fingerprint scar with a beguiling moiré pattern. The undamaged nature of this inclusion testifies to the natural, untreated origin of the gem.
B. In this sapphire from Sri Lanka, evidence of high temperature heat treatment can be found in this moiré-patterned fingerprint. The once-lovely lacy pattern of liquid droplets is now besmirched by circular “explosions,” where the pressure from heating caused ruptures in the microscopic negative crystals, thus deflowering Mother Nature’s exquisite work of art.
A. Many Mozambique rubies contain mica crystals, which are extremely heat sensitive and will be damaged by heat treatment, even at relatively low temperatures (800–1000°C). Unheated Mozambique ruby.
B. After heat treatment, Mozambique rubies with mica crystals will display glassy fissures around the mica, as shown here. Heated Mozambique ruby.
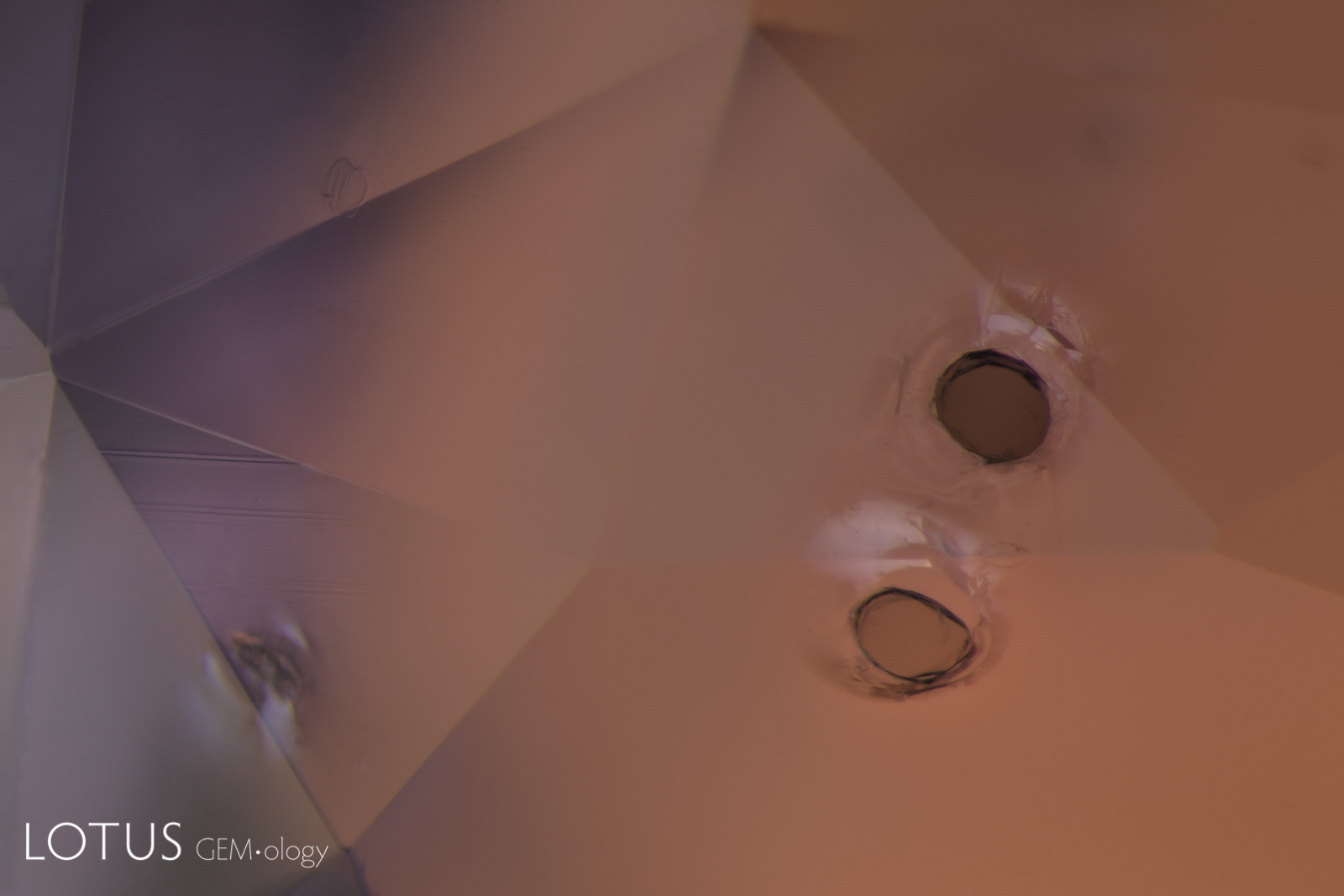
A. Many crystals contain shallow fissures on their surfaces. In sapphires from Sri Lanka, Myanmar and Madagascar, these fissures often contain epigenetic yellow stains caused by deposits from Fe-rich fluids.
B. High-temperature heating not only destroys the yellow stains, but begins a process of healing, where the fissures turn white and start forming fingerprints, as we can see here. Note that such yellow stains are generally missing in the fissures of unheated Kashmir sapphires.
A. Many rubies and sapphires contain shallow fissures on their surfaces. These typically display yellow to orange epigenetic iron oxide stains, as shown here.
B. When such a stone is heat treated, the stains turn white and bubbly fingerprints begin to appear.
A. This image clearly illustrates what master photomicrographer John Koivula has termed “chromophore cannibalization.” As titanium is drawn out of solid solution to form exsolved rutile silk, the area in the immediate vicinity is decolorized, losing its blue color. This is a natural process and is the reverse of “internal diffusion,” where heat treatment sends the titanium back into solution, creating blue “ink spot” clouds around the rutile remnants.
B. When a rutile-silk containing sapphire is artificially heated, titanium from the rutile dissolves into the surrounding sapphire. Once in solid solution, the titanium reacts with iron, creating a blue color. The result is tiny blue halos surrounding the remnants of the rutile silk, a process dubbed “ink spot internal diffusion” by John Koivula. This partially dissolved silk with blue color concentrations is a clear sign that the sapphire was heated and is the opposite of chromophore cannibalization.
A. In this heated sapphire, one can clearly see “ink spot” internal diffusion, caused when the heat treatment partially dissolves the rutile silk, sending titanium into solid solution. This is a sure sign of high-temperature heat treatment.
B. Using a powerful short-wave ultraviolet light, one can see a chalky fluorescence in the same pattern. It is the colorless, rather than blue areas, that fluoresce.
A. In this untreated sapphire from Tanzania’s Songea area, one can see a dark red, high relief crystal of primary rutile surrounded by a secondary healing halo (’rosette’).
B. When a sapphire or ruby containing either primary (shown here) or secondary rutile is heated, titanium bleeds from the rutile into the surrounding corundum (entering into solid solution), creating a blue halo. This is frequently seen in heat treated sapphires from Montana (USA), as well as beryllium-diffused sapphires from Songea (Tanzania), as shown here.
A. Unusual birefringent crystals in a low-relief partially healed fissure in a sapphire treated with high-temperature heat plus modest pressure (HT+P). When tested with Lotus Gemology’s microRaman, they proved to be corundum. It is thought that they crystallized during the treatment process. FOV 4 mm.
B. When viewed between crossed polars, the crystals stand out much more clearly. Photos: E. Billie Hughes
A. Secondary healed fissures in corundum are often filled with carbon dioxide, but usually in liquid form (solid carbon dioxide is what we know as “dry ice”). On occasion we see it in both liquid and gaseous form. This series of four images shows liquid carbon dioxide with a gas bubble (the yellow area). As the heat of the microscope warms the specimen, the gas bubble shrinks and eventually disappears. The critical temperature at which the phase changes is 31.2°C. The existence of carbon dioxide inclusions in sapphire was first noted by David Brewster in 1826. He also noted the explosive nature of such inclusions, which burst at temperatures generally between 250–400°C.
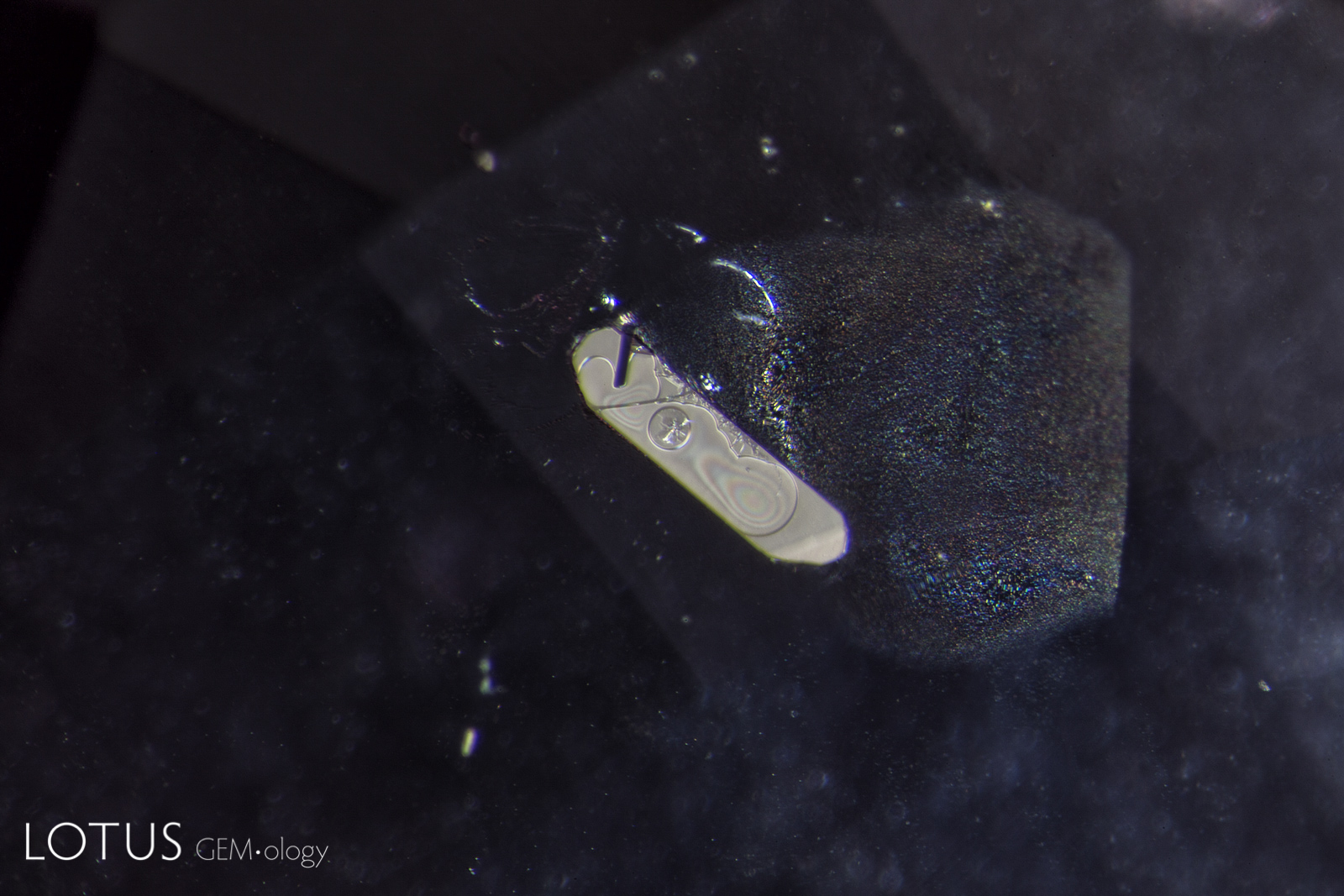
A. Rosette inclusion surrounding a mica crystal in an unheated Mozambique ruby, seen in transmitted.
B. Reflected light reveals surface detail that is masked in transmitted light.
A. Overhead lighting was used to photograph this scene in a violet sapphire from Sri Lanka, which shows two large mica plates. The undamaged state of the mica reveals that this sapphire has not been subjected to heat treatment.
B. A combination of dark field and overhead lighting was used to photograph the same scene as at left, revealing two large mica plates and smaller rounded zircon crystals. The undamaged state of the mica reveals that this sapphire has not been subjected to heat treatment.
A. In this flattened negative crystal in a Sri Lankan padparadscha sapphire, multiple phases can be found, including both liquid and gaseous carbon dioxide and a diaspore needle. Because diaspore’s refractive index (nα = 1.682–1.706; nβ = 1.705–1.725; nγ = 1.730–1.752) is so close to corundum (nω = 1.762; nε = 1.770) the diaspore needle almost disappears into the sapphire, appearing like a narrow indentation into the negative crystal. Liquid carbon dioxide becomes a gas at a fairly low temperature, with just the heat of the microscope causing the bubble to disappear. Intact negative crystals such as this are positive proof that the specimen has not been heat treated.
B. Reflected light reveals surface detail that is masked in transmitted light. Photos: Richard W. Hughes
A. A fingerprint with many small negative crystal channels showing no signs of heat-induced damage in a sapphire from Madagascar.
B. In this heat-altered fingerprint, one can clearly see that each of the negative crystal channels has burst from the heat treatment. Also note the glassy circular "discoid" fissures.
A. Brown monazite crystals are sometimes found in sapphires from Madagascar’s Ilakaka region. In this gem one can see glassy tension halos around each, indicating that the gem was subjected to low temperature (less than 1400°C) heat treatment.
B. When viewed with dark-field illumination, the glassy tension halos around each monazite crystal are more distinct. Note: Saeseaw et al. (2020; Gems & Gemology, No. 4) showed that monazite decolorizes at 600°C, suggesting that these inclusions may be natural, rather than the result of heat treatment. Monazite is radioactive, and thus that may have produced the fissures as the crystals expanded, similar to zircon in sapphire.
Note
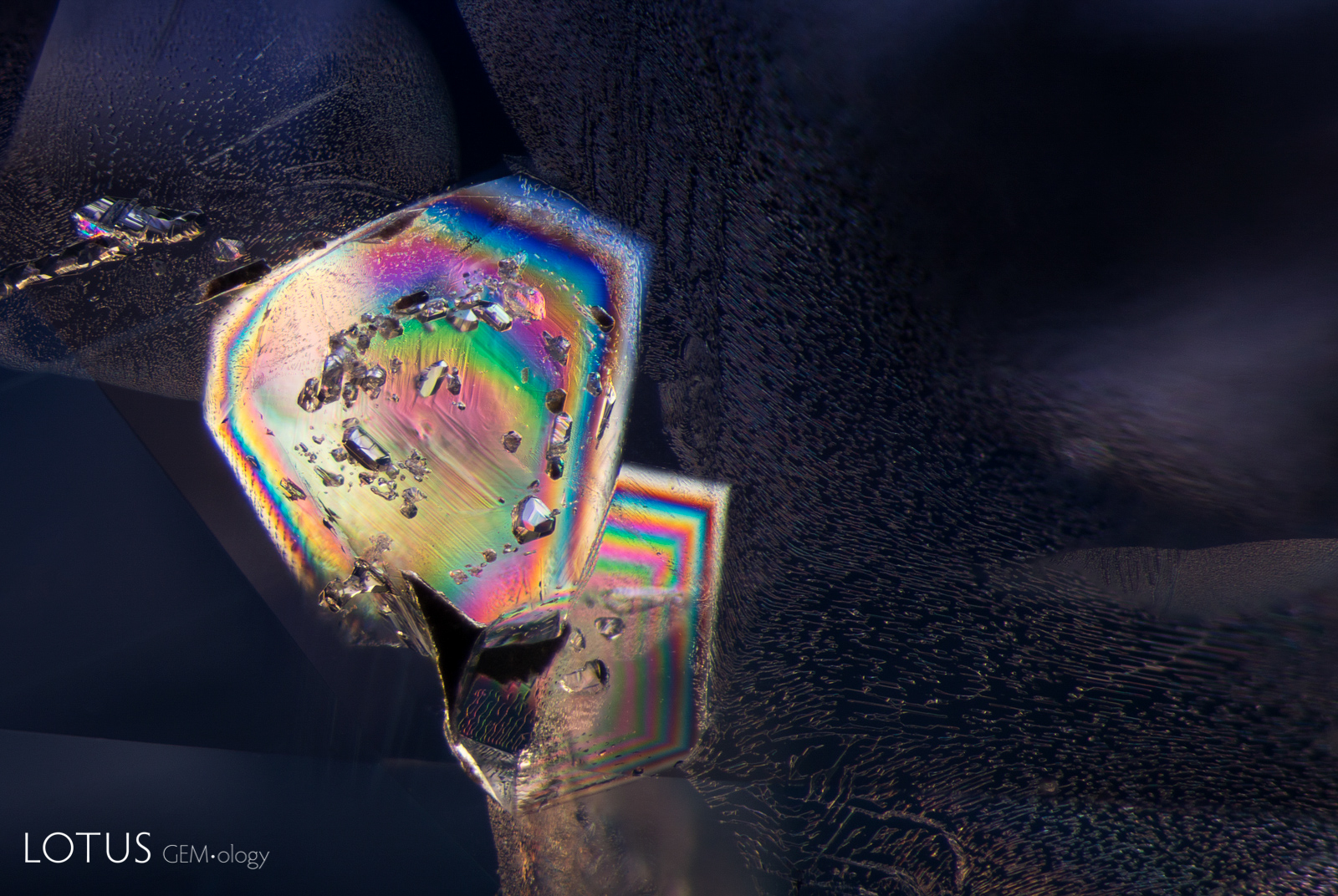
The title graphic was inspired by master photomicrographer John Koivula, who demonstrated this technique of image mirroring to create aesthetic patterns at the International Gemmological Conference in Brazil in 1987.