Distracting features in this ruby could easily cause gemologists to misidentify the stone. Learn more about how we unmasked this tricky synthetic.
The conjuror or con man is a very good provider of information. He supplies lots of data, by inference or direct statement, but it's false data.James Randi (Magician)
Introduction
The art of misdirection is essential to a good magic trick. By directing the audience’s attention towards one thing, the magician can distract from another, and then wow the crowd with a big reveal. To figure out how a magic trick is done, one must pay attention to the right details, while ignoring the distractions.
This is true in gemology as well. Recently, a great reminder of this came through our laboratory.
 Figure 1. The 17.86 ct ruby that is the subject of this report. Photo: Ronnakorn Manorotkul/Lotus Gemology
Figure 1. The 17.86 ct ruby that is the subject of this report. Photo: Ronnakorn Manorotkul/Lotus Gemology
General Properties
A client submitted a 17.86-ct ruby cabochon, which was declared as a heated Mozambique ruby (Figure 1). The stone was transparent, although with some eye-visible inclusions, which is not surprising for a ruby of such a large size. A series of standard tests was run on the stone. We noted that the stone displayed very strong red appearance in long-wave fluorescence, and a strong chalky red appearance in short-wave fluorescence, which is common for a heated ruby, although a bit unusual for a Mozambique stone (Figures 2–3). Analysis with FTIR did not reveal any diagnostic features.
A. |
B. |
| Figure 2. The ruby was examined under a standard long-wave/short-wave UV light box A. The stone displayed strong red fluorescence in long-wave UV. B. In short-wave UV, the stone displayed strong chalky red fluorescence. Photos: Ronnakorn Manorotkul/Lotus Gemology |
|
 Figure 3. The ruby was examined under a standard long-wave/short-wave UV light box A. The stone displayed strong red fluorescence in long-wave UV. B: In short-wave UV, the stone displayed strong chalky red fluorescence. Photos: Ronnakorn Manorotkul/Lotus Gemology
Figure 3. The ruby was examined under a standard long-wave/short-wave UV light box A. The stone displayed strong red fluorescence in long-wave UV. B: In short-wave UV, the stone displayed strong chalky red fluorescence. Photos: Ronnakorn Manorotkul/Lotus Gemology
Microscopic Features
With the microscope, we noted several interesting inclusions. First, there were a few clouds of very fine particles (figures 4–5) that resembled rutile silk that had been partially dissolved during high-temperature heat treatment. The stone also contained many fingerprints with white, frosty negative crystals, some with immobile gas bubbles (figure 6). Some fingerprints had drippy, melted areas (figure 7). Combined with the chalky SW fluorescence, the stone had the appearance of having undergone high-temperature heat treatment.
 Figure 4. Fine particles could be seen in the stone. Photo: E. Billie Hughes/Lotus Gemology
Figure 4. Fine particles could be seen in the stone. Photo: E. Billie Hughes/Lotus Gemology
 Figure 5. Some of the fine particles formed more dense “rain-like” streams that could superficially resemble partially dissolved silk. Photo: E. Billie Hughes/Lotus Gemology
Figure 5. Some of the fine particles formed more dense “rain-like” streams that could superficially resemble partially dissolved silk. Photo: E. Billie Hughes/Lotus Gemology
 Figure 6. The stone contained several fingerprints made up of frosty white crystals, many with immobile gas bubbles inside. These are sometimes referred to as flux “icicles.” Photo: E. Billie Hughes/Lotus Gemology
Figure 6. The stone contained several fingerprints made up of frosty white crystals, many with immobile gas bubbles inside. These are sometimes referred to as flux “icicles.” Photo: E. Billie Hughes/Lotus Gemology
 Figure 7. This melted fingerprint with drippy channels provided further evidence that the stone was heated. Photo: E. Billie Hughes/Lotus Gemology
Figure 7. This melted fingerprint with drippy channels provided further evidence that the stone was heated. Photo: E. Billie Hughes/Lotus Gemology
We took a closer look at the fingerprints, focusing on the surfaces with a fiber-optic light. Often heated rubies display some glass at the surface openings of fissures, either as a byproduct of flux heating or from glass filling, but careful examination of this stone failed to reveal this (figure 8).[1] Something was not quite right. While the ruby did look heated, the features did not seem consistent with Mozambique ruby as declared. The dotted appearance of the silk did not match the platelets we expect to see in heated Mozambique material, and the crystals looked different too.
 Figure 8. Examination of fingerprints cut through on the surface revealed no signs of glass filling. Photo: E. Billie Hughes/Lotus Gemology
Figure 8. Examination of fingerprints cut through on the surface revealed no signs of glass filling. Photo: E. Billie Hughes/Lotus Gemology
Over the years, what we have learned is that, when things don’t add up, be it gemology or any other puzzle, you are missing a key piece of information. The number one mistake in gemology is the failure to consider enough possibilities, so we widened our field of view. Could this be a synthetic? Most synthetic rubies that come through our laboratory are flame-fusion stones. These stones are generally extremely clean compared to natural stones, so their lack of inclusions is a red flag.
This stone was different. It was full of inclusions, but did not resemble either a natural ruby or the common flame-fusion synthetics. A perusal of our sample collection and Hyperion inclusion database (Hughes, 2021) suggested that it could be a flux synthetic.
This would explain the variety of inclusions. The fine particles were not partially dissolved silk, but streams of fine flux droplets, sometimes called “rain” (Hughes et al., 2017). Sometimes this can look like “comet tail” streams of fine particles, as seen in the Kashan synthetic in figure 9. The frosty white crystals were primary flux, which have been reported in Kashan synthetic rubies, and are sometimes described as flux “icicles” (Gübelin & Koivula, 2008 and Watanabe, 1987). Indeed, one of the Kashan synthetics from our sample collection contained many “icicle” type primary flux features (figure 10). It is important to note that not all primary flux inclusions in flux synthetic ruby have this whitish appearance. Some may range from orange to red in color, such as in the material produced by Ramaura (figure 11) and Douros (figure 12).
 Figure 9. “Comet” type flux inclusions in a Kashan flux grown synthetic ruby. Photo: E. Billie Hughes/Lotus Gemology Sample courtesy of AIGS
Figure 9. “Comet” type flux inclusions in a Kashan flux grown synthetic ruby. Photo: E. Billie Hughes/Lotus Gemology Sample courtesy of AIGS
 Figure 10. White, flux filled cavities in a Kashan flux grown synthetic ruby from Lotus Gemology’s sample collection. Photo: E. Billie Hughes/Lotus Gemology
Figure 10. White, flux filled cavities in a Kashan flux grown synthetic ruby from Lotus Gemology’s sample collection. Photo: E. Billie Hughes/Lotus Gemology Figure 11. Triangular growth zoning and orangey red flux crystals in a Ramaura flux grown synthetic ruby. FOV: 3 mm Photo: E. Billie Hughes/Lotus Gemology. Sample courtesy of Nicolas Francfort
Figure 11. Triangular growth zoning and orangey red flux crystals in a Ramaura flux grown synthetic ruby. FOV: 3 mm Photo: E. Billie Hughes/Lotus Gemology. Sample courtesy of Nicolas Francfort
 Figure 12. Two-phase primary flux filled cavities in a Douros flux synthetic ruby. Some areas of the cavities are transparent, but other areas are filled with a reddish orange flux. Note the angular growth zoning in the background. Photo: E. Billie Hughes/Lotus Gemology
Figure 12. Two-phase primary flux filled cavities in a Douros flux synthetic ruby. Some areas of the cavities are transparent, but other areas are filled with a reddish orange flux. Note the angular growth zoning in the background. Photo: E. Billie Hughes/Lotus Gemology
Another feature that could be misleading in flux synthetics is their growth zoning. Flux synthetics can and do have angular or straight zoning (figure 13) because they growly slowly with flat crystal faces, quite unlike the curved growth typical of flame fusion synthetics. In fact, this angular/straight zoning can appear identical to that of natural rubies.
 Figure 13. Straight growth zoning in a flux synthetic ruby. Photo: E. Billie Hughes/Lotus Gemology
Figure 13. Straight growth zoning in a flux synthetic ruby. Photo: E. Billie Hughes/Lotus Gemology
Chemistry
The chemical profiles of rubies often reveal strong evidence of their origin. Flux synthesis methods in particular tend to leave chemical clues that can be detected with various analysis techniques (Muhlmeister et al., 1998 and sources therein; Henn & Schrader, 1985; Schmetzer & Schwarz, 2007; Tang et al., 1989).
Therefore, ED-XRF was run on the stone in question and the chemical data can be summarized as follows:
| Element | Wt% (maximum detected after multiple orientations) |
|---|---|
| Al2O3 | 99.5001 |
| K2O | 0.025 |
| CaO | 0.0102 |
| TiO2 | 0.1551 |
| V2O3 | 0.0131 |
| Cr2O3 | 0.7068 |
| Fe2O3 | Below detection limit |
| Ga2O3 | Below detection limit |
| ZrO2 | 0.0033 |
|
Other: |
Below detection limit |
| Typical crucible contaminant: Pt | Below detection limit |
| Typical flux components: Na2O, MoO3, W, Pb, Bi | Below detection limit |
| Detection limits: Na–Mn = 0.1–0.001%; Fe–Bi = 0.001–0.0002% Elements not listed in table were not measured |
|
When comparing the measured data to other published data (Muhlmeister et al., 1998; Schmetzer & Schwarz, 2007), the results find good agreement with Kashan synthetic rubies. In particular, the elevated Ti levels, along with the Fe and Ga being below the detection limit, support this conclusion.
Flux synthetic rubies are often grown in platinum (Pt) crucibles; additionally, each manufacturer has preferred fluxes that are used (Muhlmeister et al., 1998; Schmetzer, 1986). These fluxes usually consist of one or more of the following: Li2O, Na3AlF6 (cryolite), Na2W2O7, MoO3, La2O3, Ta2O5, WO3, PbO, PbO4, PbF2 and Bi2O3. Hence, traces of these fluxes can be trapped in the crystal during growth and therefore can be measured with certain chemical analysis techniques.
As Kashan synthetic rubies are grown using a cryolite (Na3AlF6) flux, they typically do not contain heavy metal (Mo, W, Pb, Bi) traces that are common in the other flux growth methods. Nor do Kashan flux synthetic rubies typically contain the Pt traces (as a contaminant from the crucible) common in Chatham and Knischka flux synthetic rubies, even as platinum inclusions (figure 14). Indeed, platinum inclusions have not been reported in Kashan synthetic rubies.
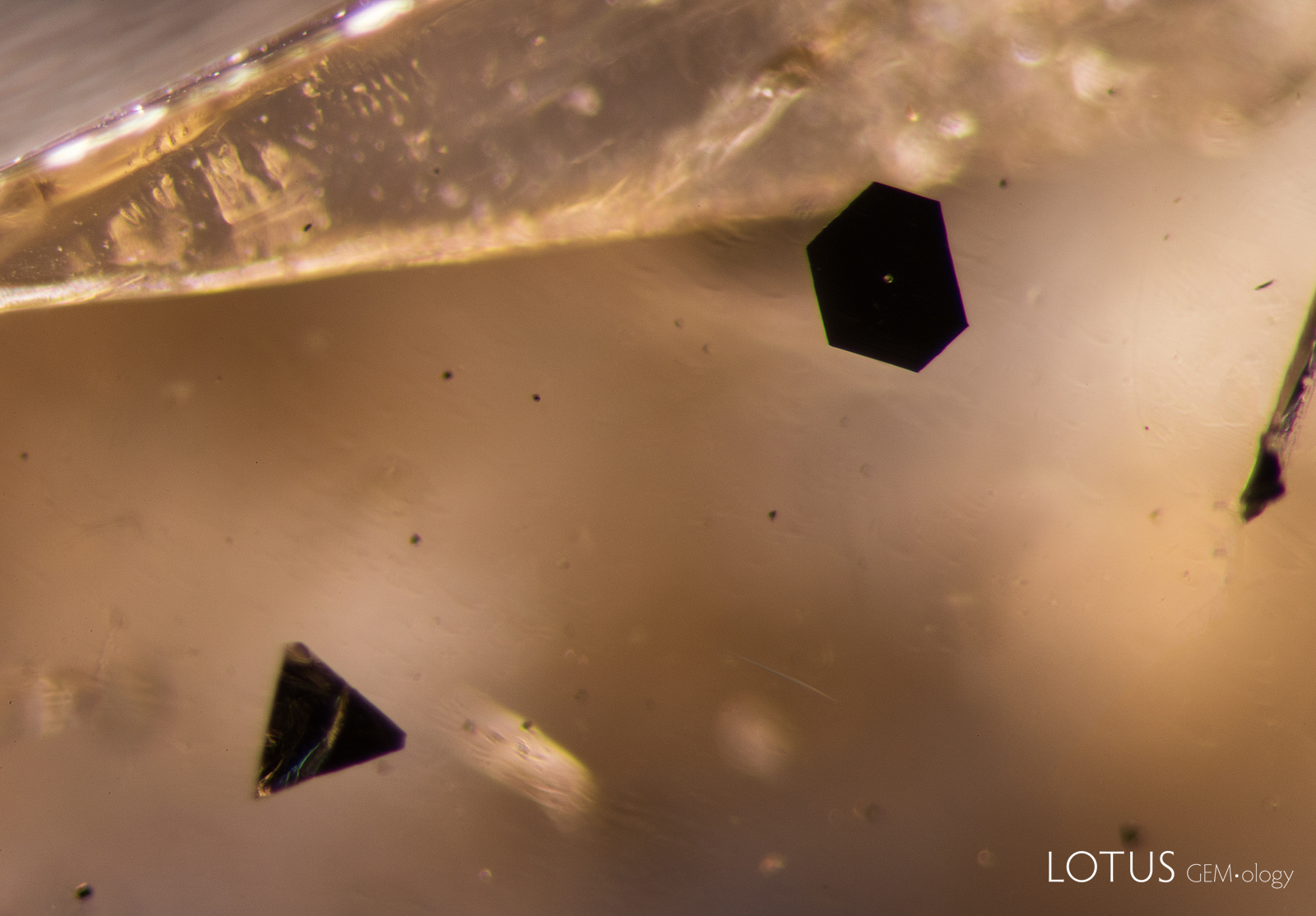 Figure 14. Triangular and hexagonal platinum platelets in a Chatham flux synthetic sapphire. Photo: E. Billie Hughes/Lotus Gemology
Figure 14. Triangular and hexagonal platinum platelets in a Chatham flux synthetic sapphire. Photo: E. Billie Hughes/Lotus Gemology
Therefore, the lack of these heavy metals being detected with ED-XRF serves as additional evidence that this synthetic ruby has been grown using the Kashan flux growth method.
Even though a Na-based flux was used for Kashan ruby synthesis, Na was not detected with ED-XRF in this case. A similar observation was made by Henn & Schrader (1985) where the Na was not detected or only in low levels with an electron microprobe. However, when neutron activation analysis (NAA) was used, the Na levels detected were relatively high. This can be explained by the differences in instrument methodology, sensitivity, sampling size and parameters employed. Thus, it is not surprising that Na was not detected in this stone with ED-XRF.
Another feature that has been reported in the literature as common in Kashan flux synthetic rubies is the elevated Ti levels (Schmetzer & Schwarz, 2007) compared to other flux synthetic ruby methods. This is believed to be the cause of Kashan stones’ slightly unusual orangish color, which is revealed by a stronger orange color in the e-ray. Indeed, this sample had Ti levels much higher than that of the Lotus Gemology flux synthetic ruby reference samples from other manufacturers.
Fluorescence
As previously mentioned, this 17.86-ct ruby displayed an extremely strong red LW fluorescence, coupled with a strong chalky red in SW (figure 2). When viewed under magnification, the chalky fluorescence was not uniform, but revealed a complex angular zoning pattern. This angularity is consistent with the growth patterns of flux synthetic ruby.
This type of chalky short-wave fluorescence was also reported in heated Kashan synthetic ruby by Singbamroong (2007).
To further compare, we observed the fluorescence of an untreated Kashan flux synthetic sample from our reference collection. In long wave, the fluorescence was a very strong red, and in short wave, it was a strong red (figure 15), without the strong chalky appearance observed in the client-submitted sample (figure 2).
Considering the combination of melted inclusions, as well as the chalky short-wave fluorescence, it was determined that this stone was heated.
A. |
B.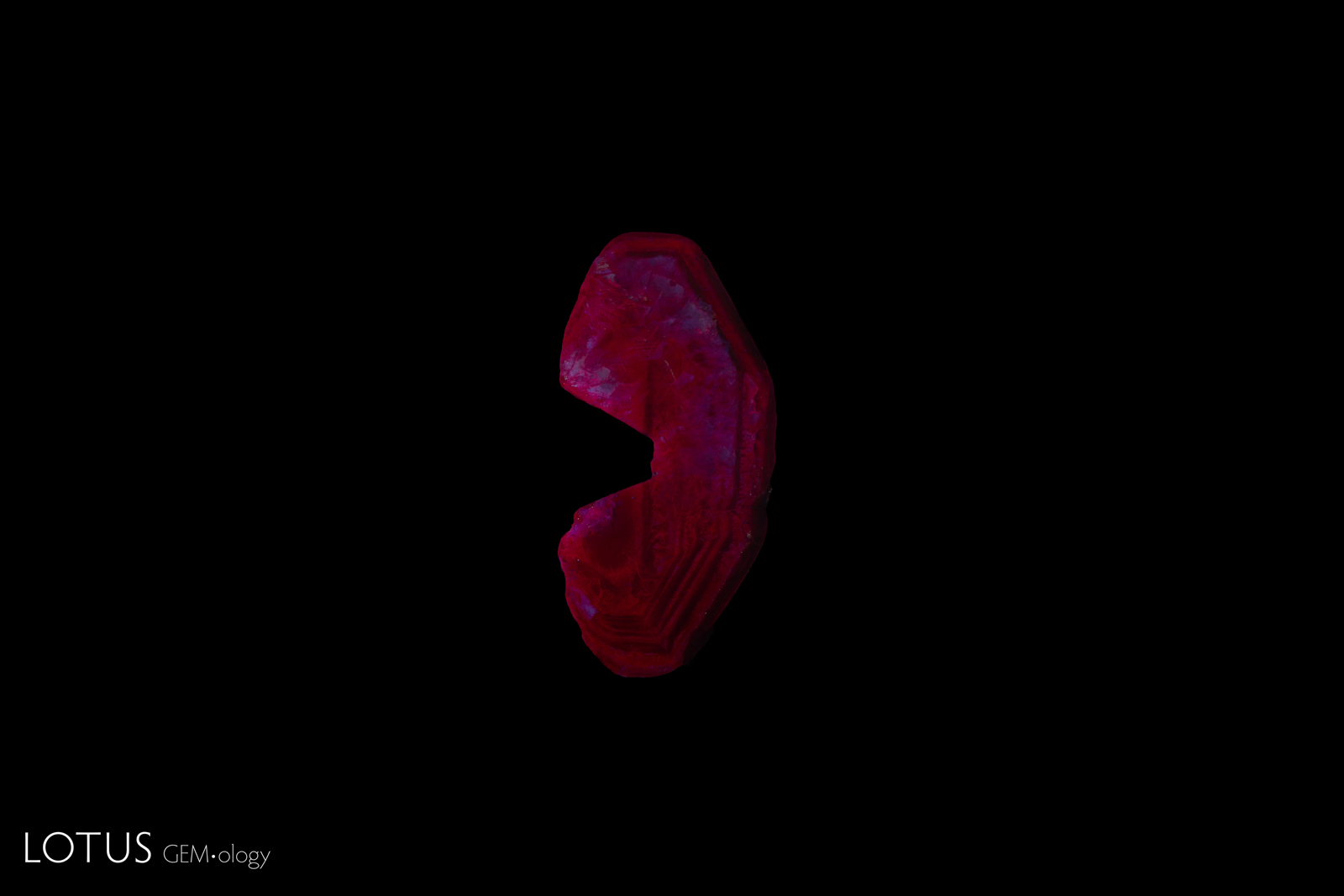 |
| Figure 15. A sample of untreated rough Kashan synthetic ruby from Lotus Gemology’s sample collection, observed under a standard UV light box. A. Under long-wave illumination (365 nm), the sample displayed a very strong red fluorescence. B. Under short-wave illumination (254 nm), the sample displayed a strong red fluorescence without a chalky appearance. Photos: Ronnakorn Manorotkul/Lotus Gemology |
|
Conclusion
Why would you heat a synthetic ruby? It’s possible that the treater thought the ruby was natural and thus heated it like they would a natural stone. The treatment could also have been carried out to obfuscate the evidence that the stone is synthetic, as has been done in the past with quench-crackled and flux-healed flame-fusion synthetics (Crowningshield, 1980 and Koivula, 1983).
While most synthetics stand out for their high clarity (and sometimes ‘too good to be true’ colors), stones like these can prove the trickiest to detect (Krzemnicki, 2016). A dusting of particles and a few melted crystals can provide enough distraction to fool a gemologist. But careful observation and thorough testing will unmask this sleight of hand.
[1] Some heat treaters will remove such glass with hydrofluoric acid after treatment (not for children!).

About the Authors
E. Billie Hughes visited her first gem mine (in Thailand) at age two and by age four had visited three major sapphire localities in Montana. A 2011 graduate of UCLA, she qualified as a Fellow of the Gemmological Association of Great Britain (FGA) in 2013. An award winning photographer and photomicrographer, she has won prizes in the Nikon Small World and Gem-A competitions, among others. Her writing and images have been featured in books, magazines, and online by Forbes, Vogue, National Geographic, and more. In 2019 the Accredited Gemologists Association awarded her their Gemological Research Grant. Billie is a sought-after lecturer and has spoken around the world to groups including Cartier and Van Cleef & Arpels. In 2020 Van Cleef & Arpels’ L’École School of Jewellery Arts staged exhibitions of her photomicrographs in Paris and Hong Kong.
Kaylan Khourie is a South African gemologist with a decade of laboratory experience focusing on diamond, corundum, beryl, tanzanite and many other gemstones. A Fellow of the Gemmological Association of Great Britain (FGA) since 2017, Kaylan has since gained a special interest in unique gems, rare synthetics and abnormal treatments. He is a big football fan and enjoys spending quality time with his family. Kaylan joined Lotus Gemology in early 2023. In 2024, Kaylan co-authored Broken Bangle • The Blunder-Besmirched History of Jade Nomenclature.
Notes
An expanded version of this article appeared in the January–June 2025 (Vol. 29, No. 1) issue of The Australian Gemmologist, pp. 67–71.
References
- Crowningshield, R. (1980) Corundum observations and problems, Gems & Gemology, Vol. 16, No. 9, pp. 315–319.
- Gübelin, E. J. & Koivula, J. I. (2008) Photoatlas of Inclusions in Gemstones, Volume 3. Basel, Switzerland, Opinio Publishers, pp.171–175.
- Hughes, E. B. (2021) Hyperion inclusion database. Retrieved January 23, 2025, from
https://lotusgemology.com/resources/hyperion-inclusion-repository - Hughes, R.W., Manorotkul, W., & Hughes, E.B. (2017) Ruby & Sapphire: A Gemologist’s Guide, RWH Publishing/Lotus Publishing, Bangkok, pp. 279–287.
- Koivula, J. (1983) Induced fingerprints, Gems & Gemology, Vol. 19, No. 4, pp.220–227.
- Krzemnicki, M. (2016) The challenge: Identification of Ramaura synthetic ruby, Facette, p. 14.
- Schmetzer, K. & Schwarz, D. (2007) The causes of colour variation in Kashan synthetic rubies and pink sapphires, Journal of Gemmology, Vol. 30, No.5/6, pp. 331–337.
- Singbamroong, S. (2007) Heat-treated Kashan grown flux synthetic ruby, Gems & Gemology, Vol. 43, No. 2, pp. 175–177.
- Watanabe, K. (1987) Inclusions in flux-grown crystals of corundum, Crystal Research and Technology, Vol. 22, No. 3, pp. 345–355.

