Jade has a long and varied nomenclatural history, with its definition altered in the current age for practical and cultural reasons. This paper examines the hardness of "fei cui" (pyroxene jade) and presents the findings in a simplified format based on empirical evidence and theoretical principles, while also considering the jade hardness data available in literature.
BACKGROUND
Several decades ago it was relatively simple to separate jadeite (a pyroxene jade rock) and nephrite (an amphibole jade rock). However the discovery that many gems labelled as "jadeite" also contain varying amounts of other clinopyroxenes (such as omphacite and kosmochlor) has complicated the situation considerably.
As with all rocks, jade is made up of an aggregate of many tiny crystals/grains; frequently these crystals/grains are made up of different minerals (see Appendix A for definitions). This makes the accurate determination of the exact end-member percentages of a rock extremely difficult and impractical. To what end should we as gemologists attempt to do this? It not only causes confusion but is also mostly irrelevant to the end-consumer.
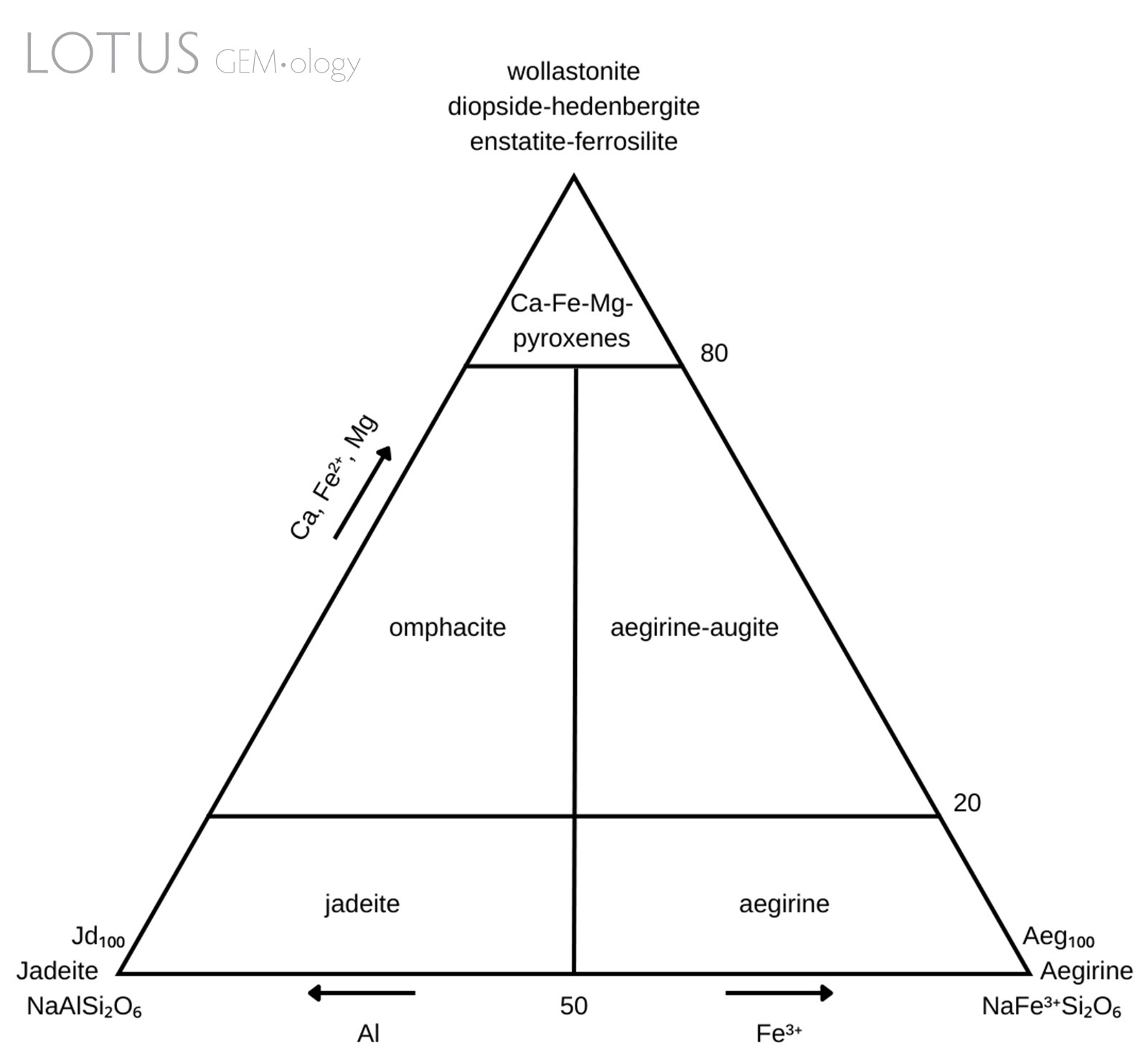 Figure 1. Pyroxene classification diagram modelled after Morimoto et al. (1988).
Figure 1. Pyroxene classification diagram modelled after Morimoto et al. (1988).
DEFINITION OF OMPHACITE
Omphacite has a complicated nomenclatural history (Clark & Papike, 1968) due to its complex chemistry and intermixing with jadeite and/or kosmochlor. Previous descriptions of a material called chloromelanite (no longer in use) match the current parameters for omphacite (Dana & Ford, 1932). Simply put, omphacite is a clinopyroxene with complicated chemistry. It has the general chemical formula (M2)(M1)[Si2O6]: the M2 cation site can consist of either Ca or Na and the M1 cation site can consist of either Al, Mg or Fe3+. The composition is intermediate within the jadeite [NaAlSi2O6]—diopside [CaMgSi2O6]—aegirine [NaFe3+Si2O6] series. There may also be minor substitution with other cations such as Fe2+, Ti4+ and Mn2+. The specific chemical parameters have been stated by Morimoto et al. (1988) and are illustrated in Figure 1.
Although not an end-member, omphacite is classified as a distinct mineral because it has a different crystal structure when compared to its related clinopyroxene members (Matsumoto et al., 1975). The space group symmetry of omphacite is related to its specific composition. Intermediate compositions have the space group P2/n, whereas the omphacites with compositions closer to diopside or jadeite end-members have the space group C2/c (Deer, Howie & Zussman, 1992). The different space groups are related to the cation ordering and may potentially exhibit measurable differences in some physical properties (Brenker, Prior & Müller, 2002).
RELATION TO JADEITE AND KOSMOCHLOR: FEI CUI
Jadeite and kosmochlor (formerly known as ureyite) are more closely related to each other than they are to omphacite because their substitutional cations occur in only the M1 site and are solely homovalent: Al3+ → Cr3+. Nevertheless, omphacite is still found in association with jadeite and kosmochlor (Franz et al., 2014; Shi et al., 2012).
Identifying a polymineralic material based on its end-member percentages is a tedious task that usually involves destroying the sample to analyze its constituents properly. Omphacite and kosmochlor are often tightly intermixed with jadeite and the point and bulk measurements on the surface are not necessarily representative of the entire sample.
Due to this, the term fei cui (pronounced like 'fay-choy') has been proposed as an umbrella term by the Gemmological Association of Hong Kong (Gemmological Association of Hong Kong, 2016) for pyroxene jades that consist of jadeite, omphacite and/or kosmochlor in varying amounts. From this point on, our pyroxene jade samples will be referred to as "fei cui" where appropriate. "Jade" will be used in reference to both pyroxene jade (fei cui) and amphibole jade (nephrite).
|
Brief History of Fei Cui Fei cui (sometimes fei tsui or fei-ts'ui) is the Chinese term that refers to the plumage of the kingfisher birds that have red (fei) and green (cui) feathers (Hansford, 1948). It is believed by Hansford (1948) that for centuries the term had been used to describe bright green nephrite due to the apparent similarity in color with the bird. However, when jadeite from Myanmar began to make its way into China, the term began to be applied solely to pyroxene jade (Hughes, 2022). The modern nomenclature of fei cui is now applied to pyroxene jade (those consisting of varying amounts of the clinopyroxene minerals jadeite, omphacite and/or kosmochlor). For more on this see From Fei Cui to Jadeite and Back. |
GEMOLOGICALLY VS MINERALOGICALLY
Gemology can be described as the bridge between science and commerce, whereas mineralogy is purely scientific. As Goddard Lenzen states in the preface to his 1970 book The History of Diamond Production and the Diamond Trade (Lenzen, 1970):
It is therefore appropriate to consider gemology not as a branch of a natural science, but simply as the knowledge of a certain type of merchandise.
For example, gemology promotes the use of varietal names that are relevant in assigning a value to the gem (i.e. ruby) but mineralogy does not allow varietal names (i.e. red corundum). Therefore, material definitions between the two fields do not always correlate. It may be mineralogically relevant to comprehensively identify a gem material based on its end-member percentages but there are always limitations to what is possible when dealing with polycrystalline (and polymineralic) rocks without destroying the sample, something that is not possible in gemology.
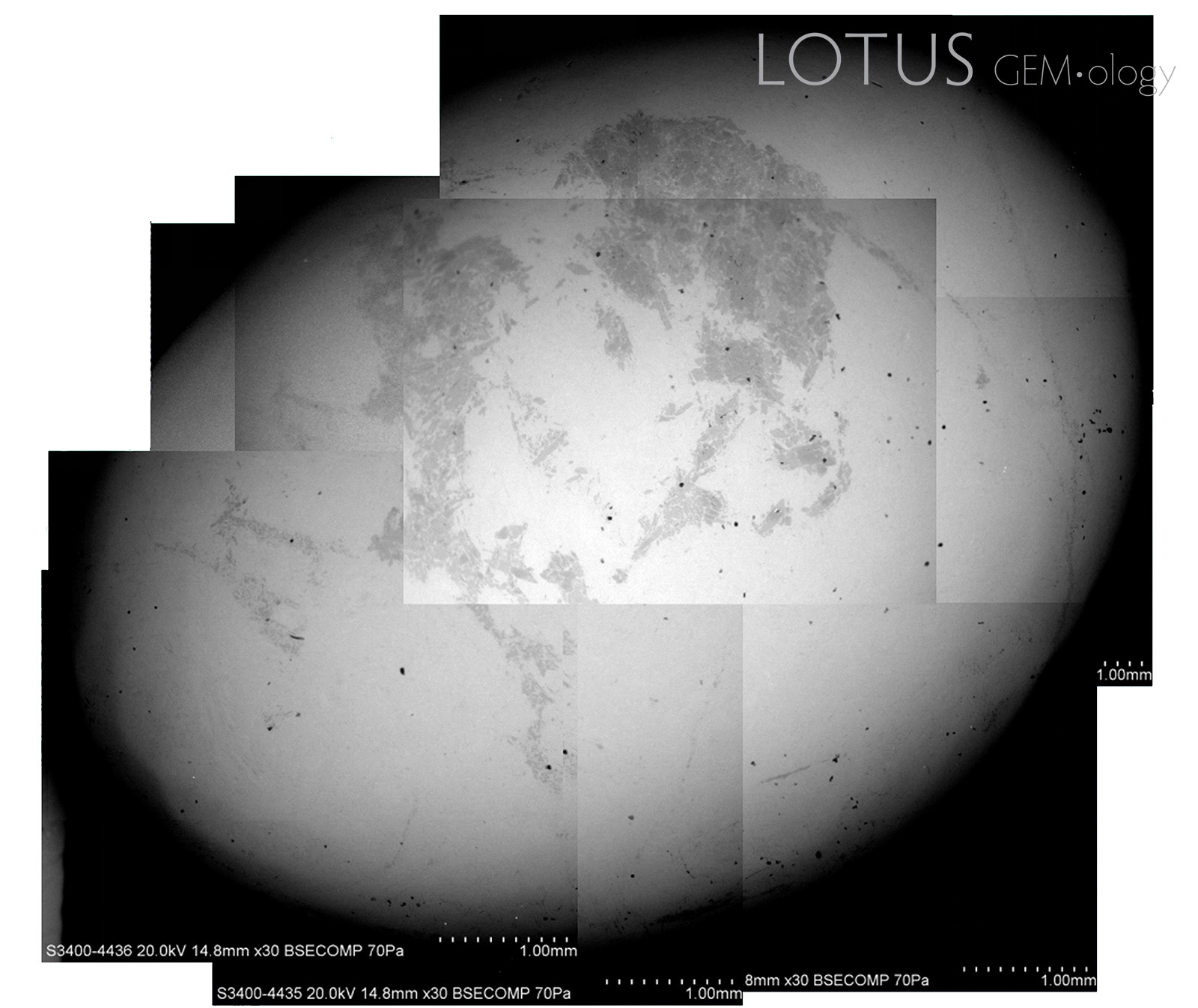
Liu, Ou Yang and Ng (2015) have illustrated the misleading results that materialize in fei cui jade testing, even when using sophisticated variable pressure scanning electron microscope (VPSEM)-Raman coupled analyses. Edward (S.I.) Liu has allowed the author to re-use their backscattered electrons (BSE) images (Figure 2).
This highlights the fact that even the most sophisticated analyses cannot comprehensively identify fei cui by its end-member percentages. Some other points to consider are:
- In most cases, only surface analyses are performed and that may not represent the entire composition of the gem material.
- Surface roughness can also affect measurement accuracy (Hernández-Murillo et al., 2022) and some determinations rely on small changes in peak position and shape. Many fei cui samples do not have a perfect polish and so this can be problematic.
- Some mineral grains are so tiny that spectroscopy will only reveal the spectrum of the dominant mineral in the beam spot or an intermediate spectrum (Figure 3).
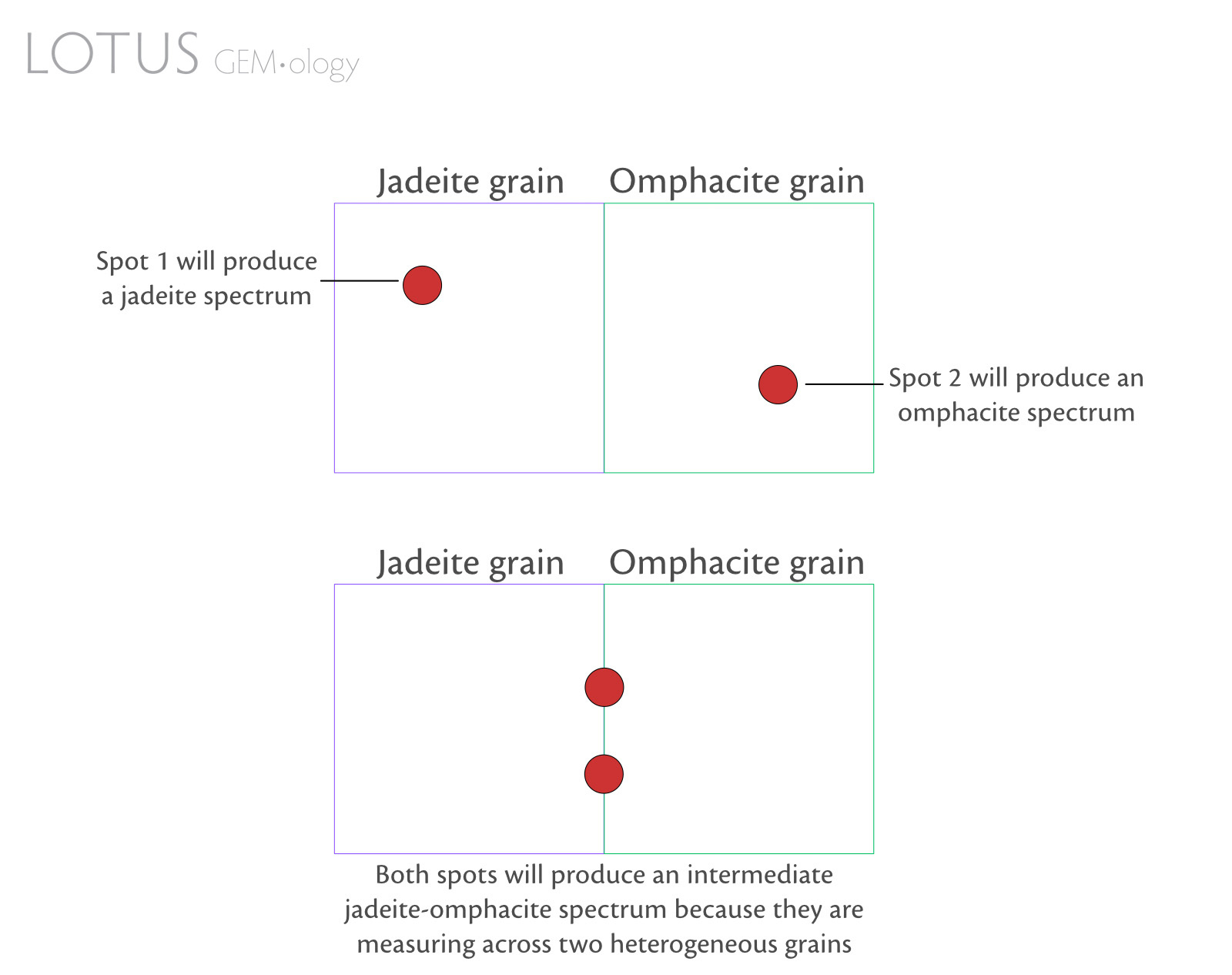 Figure 3. Simple diagram to illustrate how adjacent inhomogeneous pyroxene grains in fei cui jade can influence spectral output.
Figure 3. Simple diagram to illustrate how adjacent inhomogeneous pyroxene grains in fei cui jade can influence spectral output.
DEFINITION OF HARDNESS (SCRATCH RESISTANCE VS MICROINDENTATION)
Young (1961) states in The Microhardness of Opaque Minerals that:
Hardness has been given many definitions, but it is impossible to define it in precise terms because of the number of different physical properties which it embraces.
It was therefore advised by Young to use Ashby's (1951, pp. 33) definition:
Hardness is a measure of the resistance to permanent deformation or damage.
Scratch resistance is typically derived using the well-known Mohs scale by traversed indentation of a material of a known Mohs hardness. This is a relative hardness measurement technique.
Microindentation hardness, using techniques like Vickers microindentation testers, provides an absolute hardness value; but the values depend on the load being applied (amongst other factors).
There are many factors that affect the measured Mohs and Vickers hardness values of a material. This is made more complicated by testing heterogeneous polycrystalline materials. These factors include load, load time, indentation depth, surface roughness, grain size, grain boundaries, fibrosity, defects, chemical composition, space groups, crystallographic orientation, and even a surface compaction phenomenon detailed by Young (1961) called 'work hardening' caused by the polishing process. All these can affect the measured hardness of materials. Craig and Vaughan (1994) detail many of these factors.
Hardness is defined by Dorner and Stöckhert (2004) as a solid's resistance to local (at the point of contact) deformation. Permanent (inelastic) damage to a material after an applied force (stress) is termed plastic deformation and can be simplified to the breaking of chemical bonds that result in the slipping of dislocations which causes the material to 'shift'. The stronger the chemical bonds, the more difficult they are to break. For example, diamond's high hardness results from its covalent tetrahedrally bonded carbon atoms in three dimensions, which are extremely strong.
Young (1961) suggests that hardness is the strength of the weakest bonding present in the material, however this would be extremely difficult to quantify in heterogeneous samples. The strength of a bond is related to the combination of the distance between the anion and cation, the valency and the bonding type.
Broz, Cook and Whitney (2006) note that there is a difference between hardness and scratch resistance. Scratch resistance (Mohs) is not only dependent on hardness; it is a complex function of hardness (resistance to inelastic deformation), fracture toughness (resistance to fracture), elastic modulus (resistance to elastic deformation) and the loading method. This has been experimentally supported by the studies of the scratch resistance and hardness of glass (Sawamura & Wondraczek, 2018).
Sawamura and Wondraczek (2018) show that, in their study, the variance in the ratio of scratch resistance and hardness indicates the main difference between the two. They show that scratching generally requires higher effort to deform a material than indentation. This is due to the discrepancies in friction and material pileup. A scratch applies force both parallel to the normal and laterally, whereas indentation is only parallel to the normal.
Lawn and Marshall (1979) discuss the difference between indentation hardness and fracture toughness. When sufficient force is applied to a brittle material it will generally first deform (hardness) and then fracture (toughness) as the force increases.
Bradt, Newnham and Biggers (1973) studied the toughness of jadeite and nephrite. Toughness is a major contributing factor to the durability of a gem material, however investigating the toughness of the fei cui minerals is beyond the scope of this study.
MATERIALS AND METHODS
 Figure 4. All 22 samples of jadeite, omphacite, kosmochlor, nephrite, chalcedony and quartzite in this study. Note that the identifications of the pyroxene (fei cui) jades were made by multi-spot spectroscopic and EDXRF chemical analyses, which do not necessarily reflect the absolute compositions of the samples. True compositions can only be accurately determined by destructive testing. Photos: Ronnakorn Manorotkul/Lotus Gemology.
Figure 4. All 22 samples of jadeite, omphacite, kosmochlor, nephrite, chalcedony and quartzite in this study. Note that the identifications of the pyroxene (fei cui) jades were made by multi-spot spectroscopic and EDXRF chemical analyses, which do not necessarily reflect the absolute compositions of the samples. True compositions can only be accurately determined by destructive testing. Photos: Ronnakorn Manorotkul/Lotus Gemology.
The 22 samples chosen for this study are visible in Figure 4. They were obtained through secondary sources (dealers, colleagues, markets). The samples were first polished to have two parallel flat surfaces. They were then analyzed using the following instruments:
- Reflectance spectra: Bruker Tensor 27 FTIR with a Pike UpIR attachment.
- Raman spectra: MAGI GemmoRaman-532.
- Chemical analyses: Skyray EDX 6000B EDXRF set up with condition and curve parameters specific to jade.
- RIs and hydrostatic SGs were also measured.
The samples were then sent for Vickers microindentation measurements at the NSTDA Characterization and Testing Service Center (NCTC) in Bangkok. Subsequently, the samples were tested using a Mohs hardness pencil set.
 |
 |
|
Figure 5. Left: Shimadzu Microhardness Tester HMV-G31-FA-D-HC60. Right: Example of a Vickers microindentation. FOV: 0.2 mm. Photos courtesy of NCTC. |
|
Tan et al. (1978) used the microindentation method suggested by Hutchinson (1974) to obtain hardness data on their nephrite samples from Hualien, Taiwan. They found a range of hardness data for their samples and found that their nephrite samples correlated to Mohs 4.1 to 7.0 depending on crystallographic orientation. The nephrite samples included in this study, that were used as references, showed good correlation with the data from Tan et al. (1978). Therefore, the methods suggested by Hutchinson (1974) were taken under advisement when choosing the specific indentation measurement parameters for this study.
Out of the 22 samples in this study, focus was assigned to these four representatives:
- Jadeite: 7809-Jd
- Omphacite: 7842-Omp
- Omphacite-Jadeite mix: 7838-Omp(Jd)
- Kosmochlor: 7307-Kos
These samples were selected for focus because they represent this study's 'purest' sample of each fei cui mineral (based on the measurable data obtained). Sample 7838-Omp(Jd) is the closest representative to a 50/50% mix of jadeite and omphacite. The identifications are based on the EDXRF, multi-spot Raman and multi-spot reflectance FTIR measurements that were obtained. Even though, as mentioned above, there is currently no scientifically accurate way to determine the total composition of a rock such as fei cui, these combination analyses allowed a general idea of the measurable surface components present in the samples. The results of the other samples were also considered.
Note that another kosmochlor sample cut from the same rough as the one in this study was analyzed using SEM-EDS by Edward Liu and contained trace amounts of chromite (Fe2+Cr3+2O4). This may affect some of the measured data of the sample, such as EDXRF.
RESULTS
The results of our testing are summarized below with focus on the four representative samples.
CHEMISTRY
| Table 1. Chemical composition of fei cui samples based on EDXRF measurements. These analyses may not represent the true chemical composition of the samples because EDXRF is only semi-quantitative. | ||||||||
| Sample (wt. %) | Na2O | MgO | Al2O3 | SiO2 | CaO | Cr2O3 | Fe2O3 | Trace |
| 7809-Jd | 12.76 | 0.00 | 25.21 | 60.93 | 0.67 | 0.00 | 0.35 | 0.07 |
| 7838-Omp(Jd) | 9.68 | 3.48 | 15.17 | 59.81 | 8.20 | 0.00 | 2.61 | 1.05 |
| 7842-Omp | 4.36 | 9.98 | 11.34 | 56.74 | 13.15 | 0.00 | 3.43 | 0.99 |
| 7307-Kos | 5.12 | 1.33 | 4.97 | 39.55 | 4.12 | 41.53 | 2.47 | 0.92 |
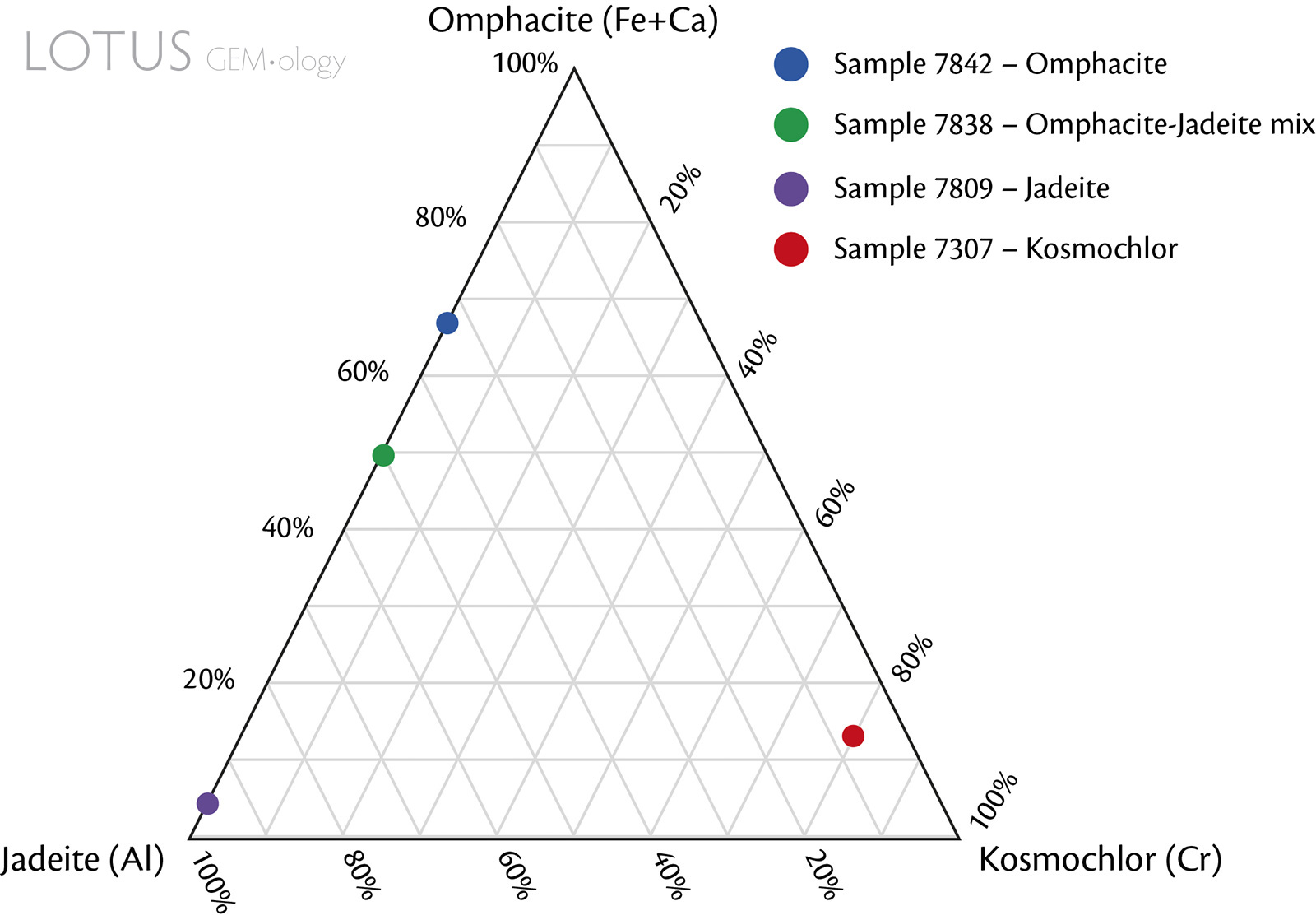 Figure 6. Compositional ternary plot of the fei cui jade samples. Mineral compositions were normalised by the main associated cation (or combination of cations) based on EDXRF data. Note that the EDXRF chemical analyses are limited because they are only semi-quantitative.
Figure 6. Compositional ternary plot of the fei cui jade samples. Mineral compositions were normalised by the main associated cation (or combination of cations) based on EDXRF data. Note that the EDXRF chemical analyses are limited because they are only semi-quantitative.
SPECTROSCOPY
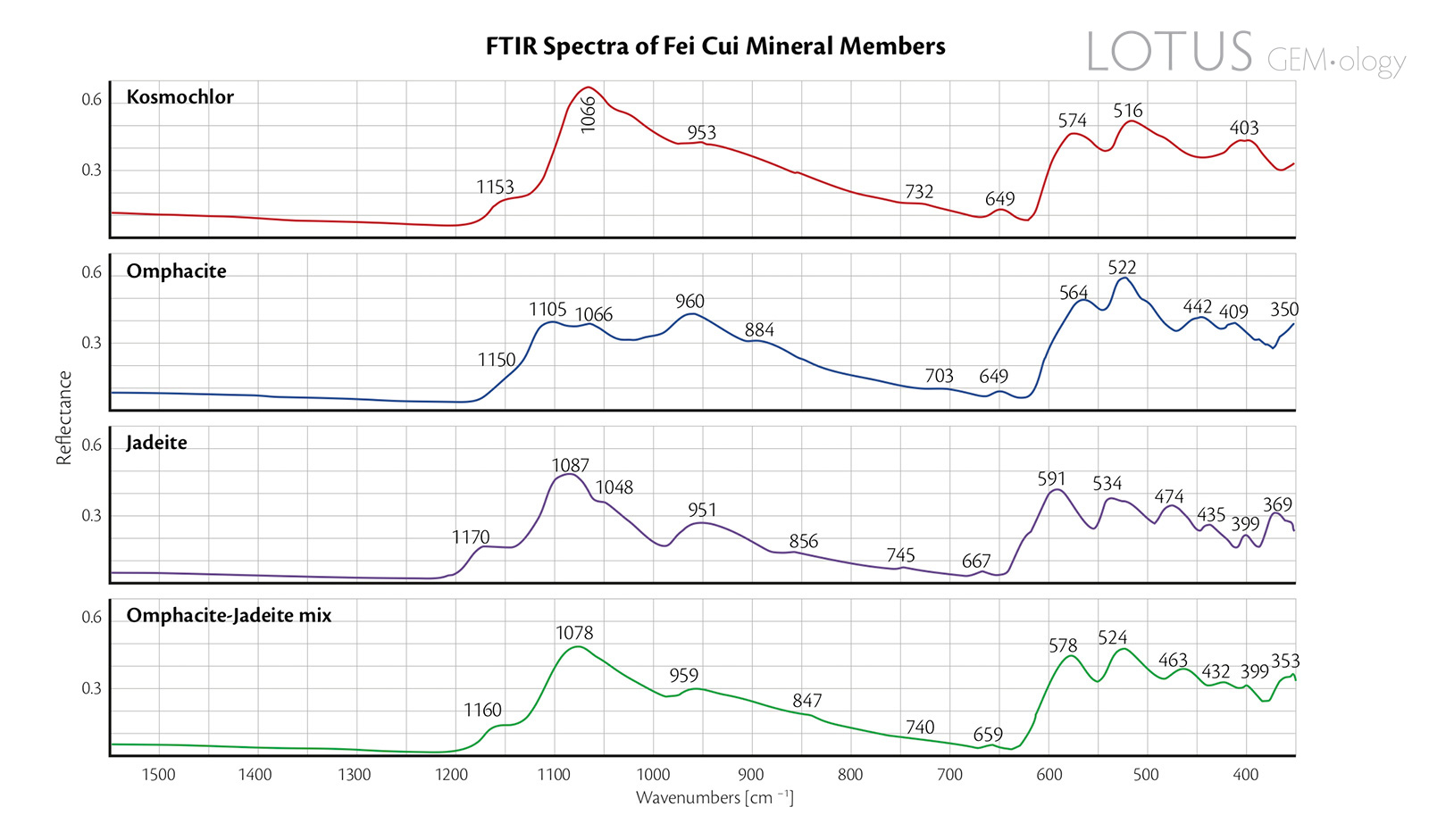 Figure 7. Reflectance FTIR spectra of the four representative fei cui jade samples.
Figure 7. Reflectance FTIR spectra of the four representative fei cui jade samples.
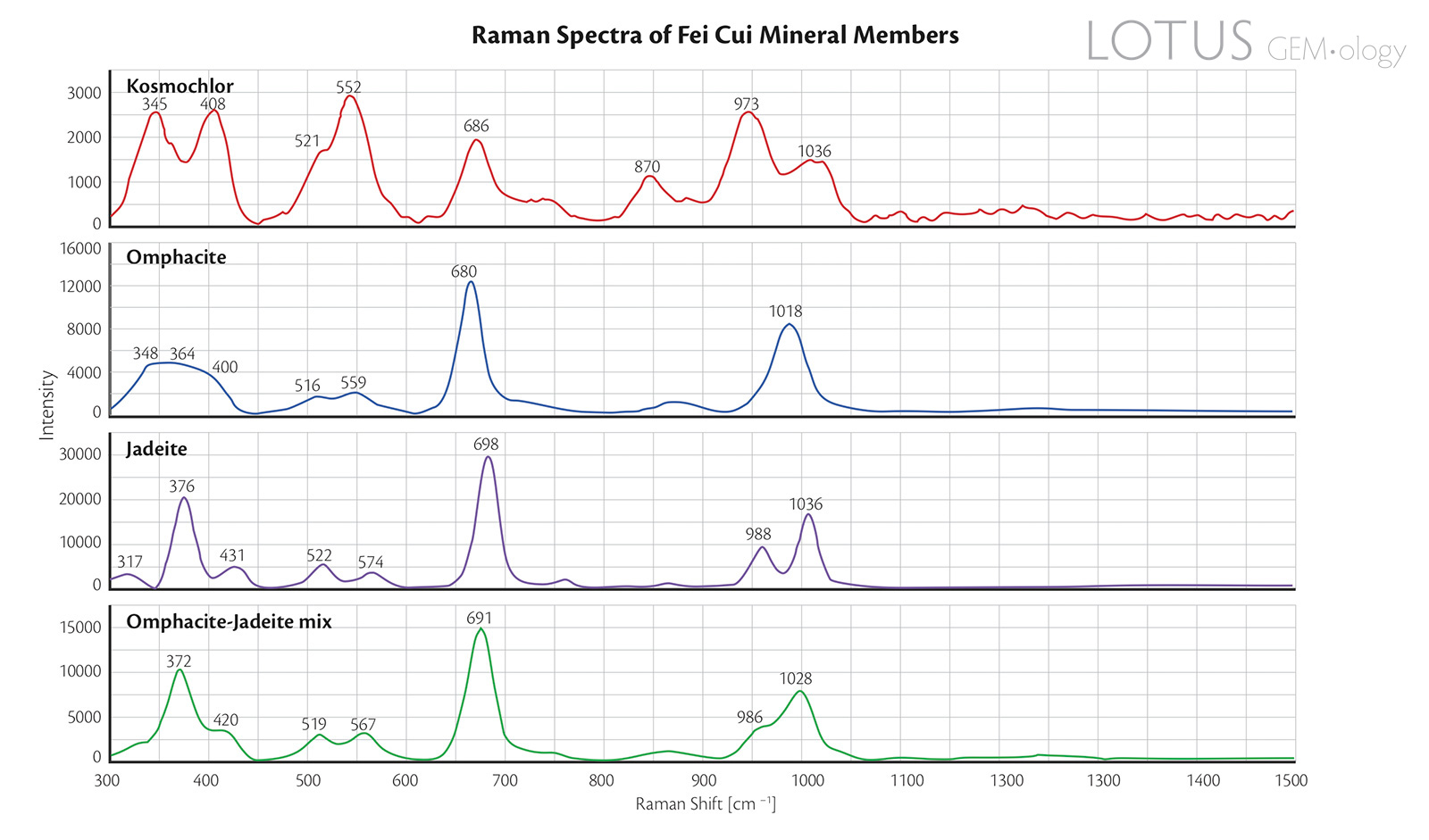 Figure 8. Raman spectra of the four representative fei cui jade samples.
Figure 8. Raman spectra of the four representative fei cui jade samples.
VICKERS MICROHARDNESS RESULTS
| Table 2. Vickers microhardness values (in GPa) per indentation spot. Sorted according to the average values across the 5 spots. | |||||||||
| Sample (GPa) | ID | 0.49N | 0.98N | 1.47N | 1.96N | 2.94N | Max | Min | Average |
| 7842-Omp | Omphacite | 9.67 | 9.64 | 8.67 | 9.45 | 9.83 | 9.83 | 8.67 | 9.45 |
| 7838-Omp(Jd) | Omphacite-Jadeite mix | 10.02 | 9.51 | 10.15 | 8.38 | 10.32 | 10.32 | 8.38 | 9.68 |
| 7840-Qz | Quartzite | 10.41 | 10.47 | 11.92 | 10.85 | 11.64 | 11.92 | 10.41 | 11.06 |
| 7809-Jd | Jadeite | 12.92 | 12.19 | 10.05 | 9.90 | 10.72 | 12.92 | 9.90 | 11.15 |
| 7307-Kos | Kosmochlor | 11.22 | 10.97 | 11.64 | 11.50 | 11.45 | 11.64 | 10.97 | 11.36 |
The Vickers microhardness (HV) was measured using five different indentation loads to account for the indentation size effect (ISE) that links the measured microhardness of a material to the load used. A lower load usually results in a higher hardness measurement and vice versa (Petrik, 2016). As there is not a standardized load suggested for clinopyroxenes, a range from 50g (0.49N) to 300g (2.94N) was used. The microhardness measurements are summarized in Table 2 and have been converted into GPa to correspond to the newtons (N) of force for each indentation spot (1 HV = 0.009807 GPa). The values of the quartzite sample have been included for comparative reasons discussed later in this article.
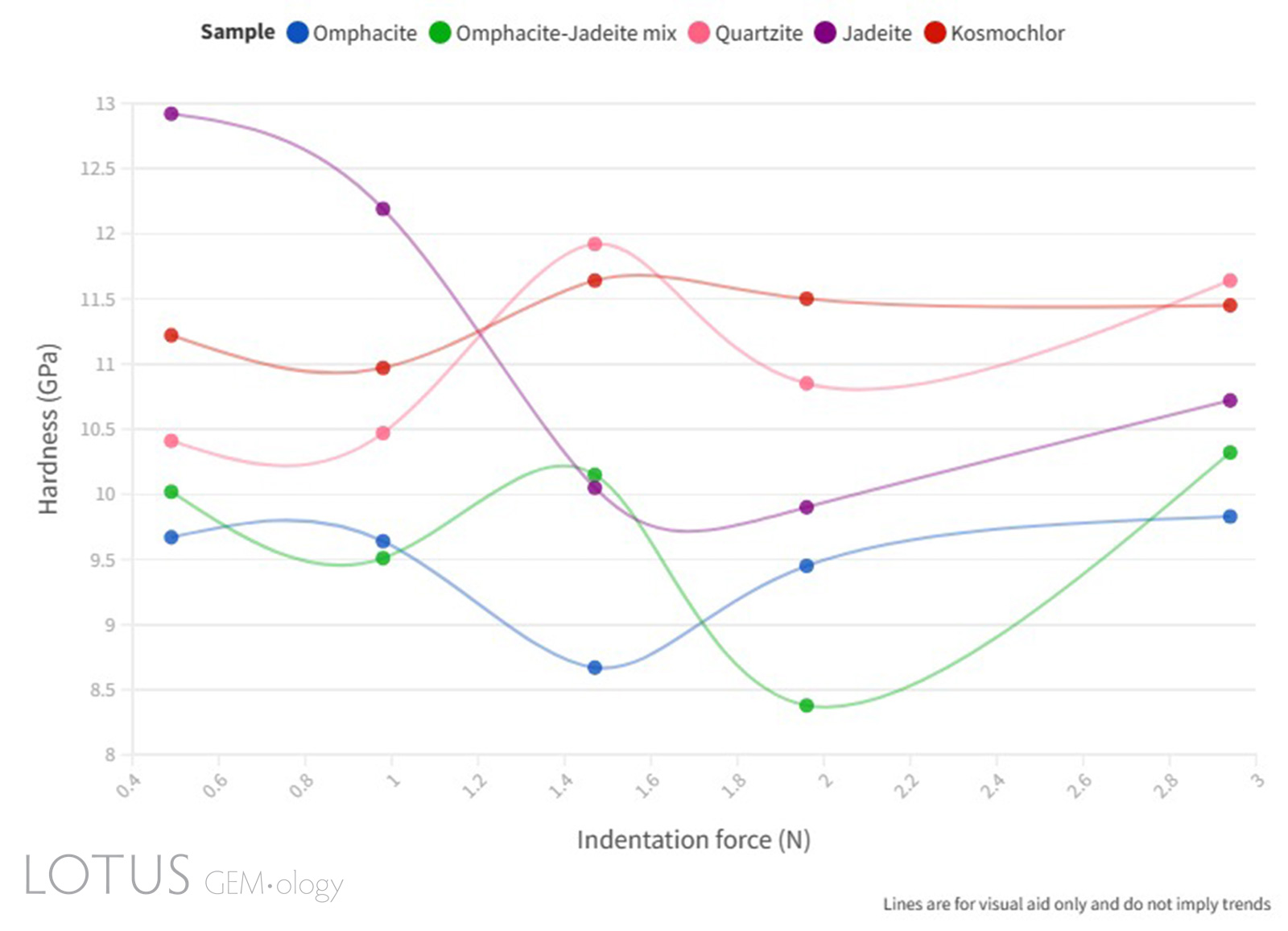 Figure 9. Line graph illustrating the Vickers microhardness (GPa) vs indentation force (N) relationship of the quartzite and fei cui jade samples.
Figure 9. Line graph illustrating the Vickers microhardness (GPa) vs indentation force (N) relationship of the quartzite and fei cui jade samples.
Click here to view an interactive version of Figure 9.
As can be seen in Figure 9, a complete ISE was not observed in the samples measured, this is assumed to be due to the complex factors that affect the indentation hardness measurements of polycrystalline materials. The microstructural effect on the microhardness of ceramics investigated by Sargent and Page (1978) confirms this assumption and their studies further explain that the relationship between microhardness and grain size is likely due to the weakening of grain boundaries.
Additionally, Voyiadjis and Yaghoobi (2017) used indentation depth to compare the difference between the microhardness of single crystal and polycrystalline aluminum samples. Inhomogeneity likely also plays a role.
COMPARISON TO VALUES REPORTED IN LITERATURE
Tan, Ng and Lim (2013) measured the microindentation hardness of jadeite and found the Vickers hardness of their samples to be 1014± 69 HV, which equals 9.94± 0.68 GPa. However, because the load of the measurement was not stated, their findings cannot be directly compared to the ones in this study.
Although Dorner and Stöckhert (2004) measured the microhardness of jadeite and diopside (the end-members of omphacite), they did so at elevated temperatures and consequently their results cannot be directly compared to the ones in this study either.
Thus, the measurements of this study represent the novel value range for Vickers microhardness measurements at different loads for fei cui minerals at room temperature.
 Figure 10. A Mohs hardness scratch testing kit. Photo: Chanon Yimkeativong/Lotus Gemology.
Figure 10. A Mohs hardness scratch testing kit. Photo: Chanon Yimkeativong/Lotus Gemology.
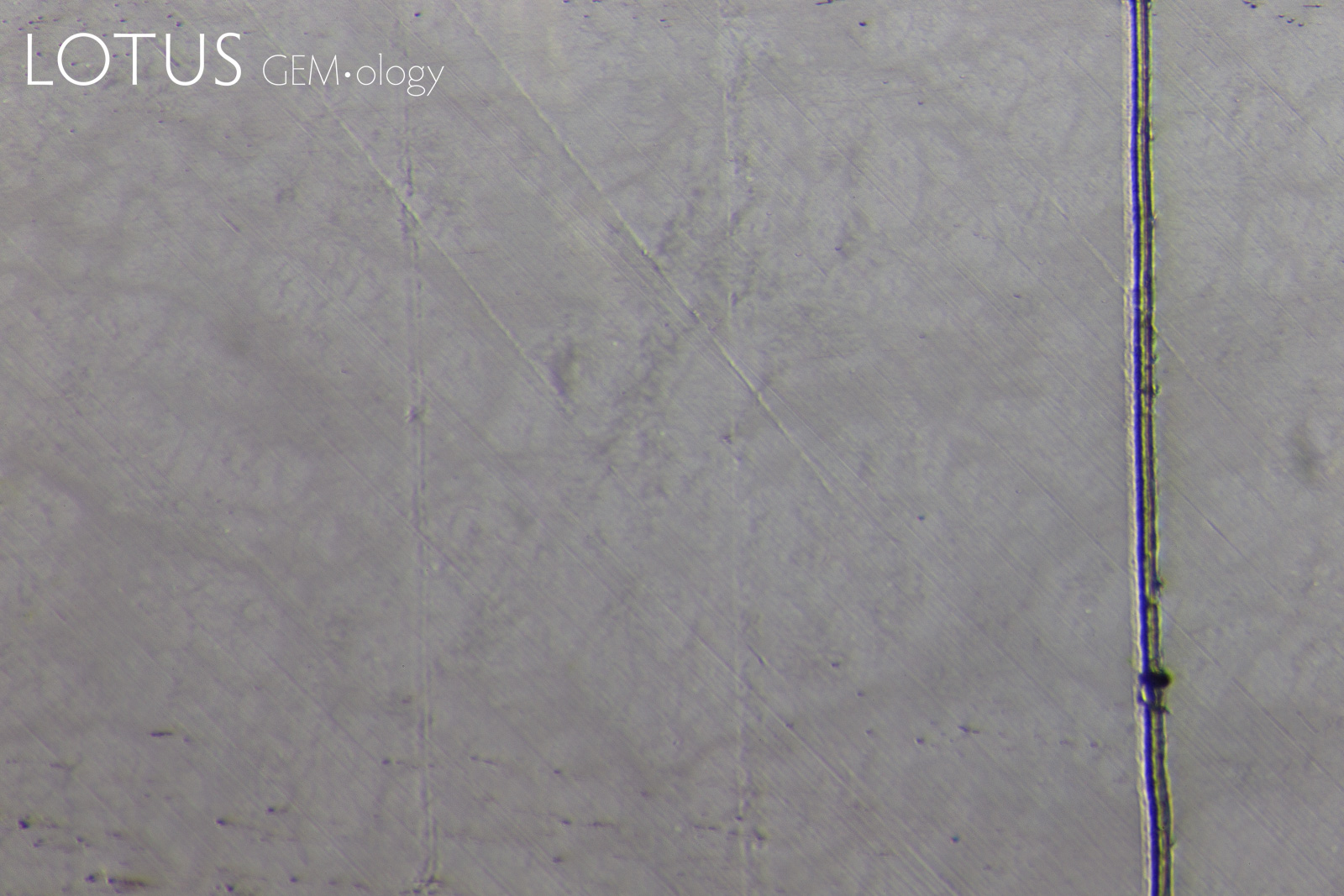
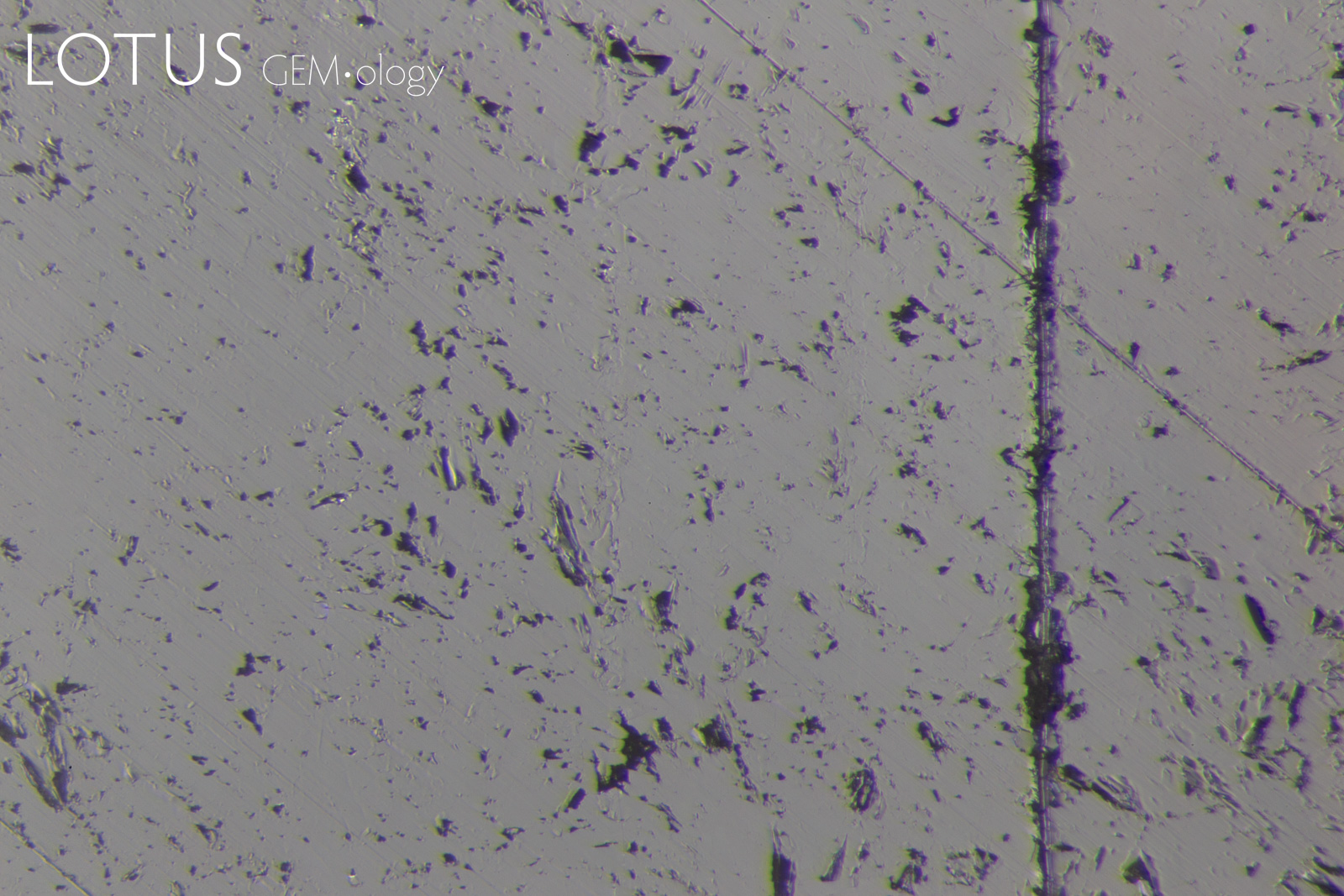
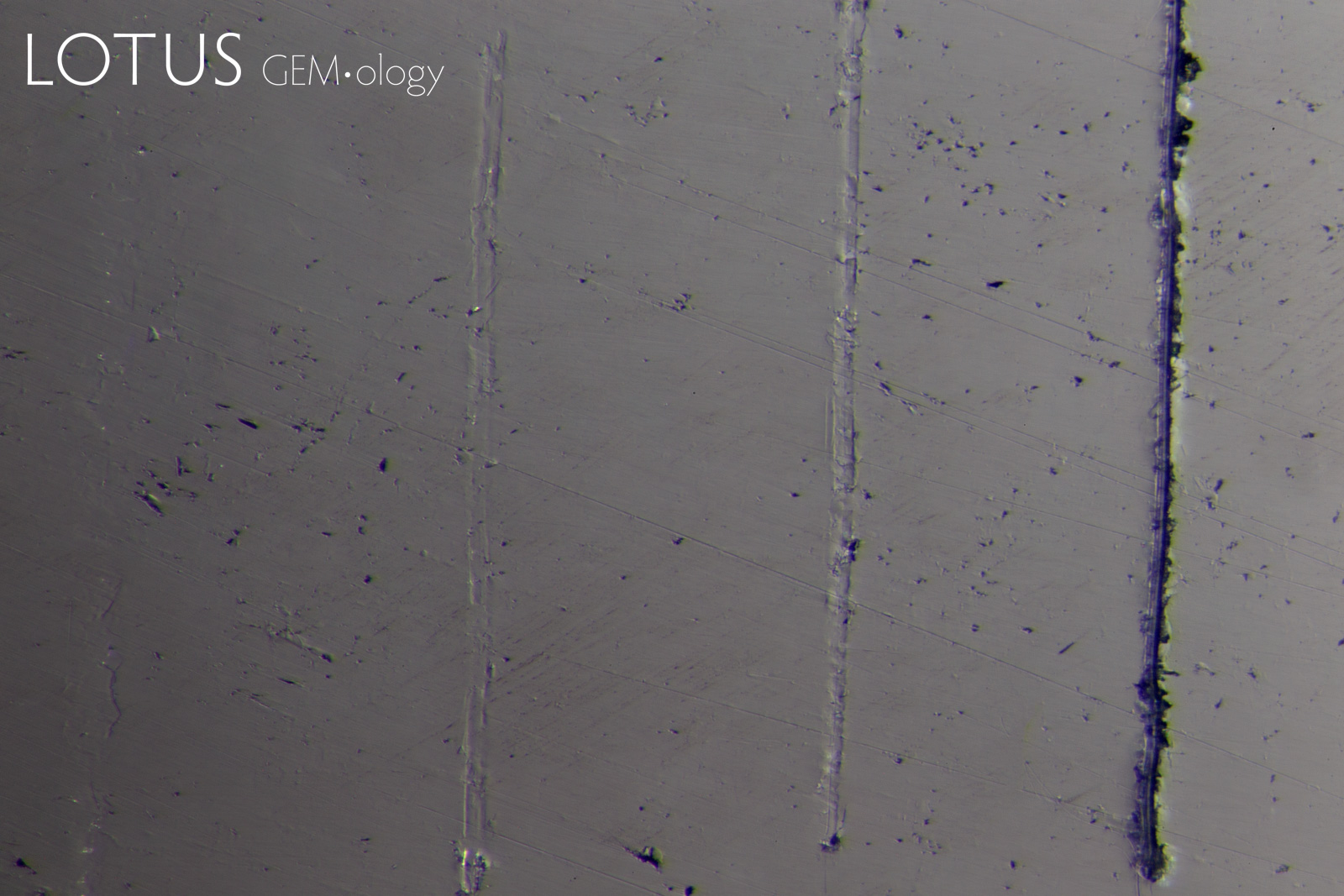
PHOTOMICROGRAPHS OF MOHS TESTING
DISCUSSION
Tabor (1954, pp. 252) states that "scratching is associated with a 'biting-in'; non-scratching with a 'skidding-over'". As can be seen in Figure 11, sample 7842-Omp displays a 'skidding over' appearance when using a Mohs 5 pencil and a 'biting-in' appearance with a Mohs 6 pencil. Therefore, this omphacite sample could be labelled as Mohs hardness 5.5.
Interestingly, sample 7838-Omp(Jd) exhibits more deformation with a Mohs 6 pencil than it does with a Mohs 7 pencil, the author could find no reports of this anomalous observation in other publications at the time of investigation. This phenomenon was observed with many of the other fei cui samples.
Sample 7809-Jd exhibits a 'skidding-over' appearance up to the Mohs 7 pencil but is severely scratched by the Mohs 8 pencil. This can then be described as a Mohs hardness 7.
Although sample 7307-Kos was severely scratched by the Mohs 8 pencil, it was not as severe as the other fei cui samples. This may indicate a slightly higher scratch resistance. However, similarly to sample 7838-Omp(Jd), the Mohs 6 pencil deformed the sample slightly more than the Mohs 7.
More than 200 years have passed since Friedrich Mohs published his scale on scratch hardnesses of minerals and there is still not a complete agreed understanding of the Mohs system (Gerberich et al., 2015). As some of the samples in this study did not show a linear degree of scratch deformation with increasing Mohs hardness points, it supports the idea that Mohs scratch testing on polycrystalline heterogeneous samples is not always straightforward. Indeed, there have been discrepancies in the reporting of the Mohs hardness of fei cui (see Table 3).
| Table 3. Mohs hardness of fei cui minerals as reported in the literature | |||||
| Mineral | Dana & Ford, 1932 |
Deer, Howie & Zussman, 1992 |
Ou Yang et al., 2003 |
Adamo et al., 2006 |
GAHK, 2016 |
| Jadeite | 6.5–7 | 6 | — | — | 6.5–7 |
| Kosmochlor | — | 6 | — | — | 5.5–6 |
| Omphacite | — | 5–6 | 7 | 6.5 | 7 |
Dorner and Stöckhert (2004) discovered that although their omphacite samples fell within the intermediate compositional range of jadeite and diopside, the mechanical data did not correlate to the intermediate of their end-member samples' mechanical data. This led them to the suspicion that inhomogeneity of the omphacite samples could be a possible reason for the unexpected variability in their hardness.
In the studies of Tan, Ng and Lim (2013), a calibration curve was determined for relating Vickers microindentation values to Mohs hardness values. Following this curve, the HV and GPa values of some of the Mohs scratch pencils can be estimated (see Table 4).
|
Table 4. Mohs vs HV vs GPa based on the calibration curve of Tan, Ng and Lim (2013). |
||
| Mohs | HV | GPA |
| 5 | 550 | 5.4 |
| 5.5 | 650 | 6.4 |
| 6 | 750 | 7.3 |
| 6.5 | 950 | 9.3 |
| 7 | 1100 | 10.8 |
Broz, Cook and Whitney (2006) measured the indentation hardness of the Mohs minerals; however, there is a better correlation between the measurements of Tan, Ng and Lim (2013) and the quartzite sample in this study.
Mukhopadhyay and Paufler (2006) show how the relationship between grain size and hardness is non-linear in certain materials. This could be a contributing factor to the microindentation measurements that were obtained. Another issue with measuring the hardness of fei cui is the inhomogeneity. For example, spot one could be measured on a large jadeite grain and spot two on a small kosmochlor grain, this could unfortunately skew the results.
Interestingly, Htein and Naing (1995, pp. 317) observed that in practice the Mohs hardness of pyroxene jade varies from "slightly greater than 6" for coarse-grained aggregates to 7 for fine-grained aggregates.
INFLUENCE OF CHEMICAL BONDING ON HARDNESS
Cameron et al. (1973) investigated the thermal expansion coefficients of the different bonding present in clinopyroxenes. Based on their studies, they stated that the following series is a function of decreasing bond strength:
Si4+-O > Cr3+-O > Fe3+-O > Al3+-O > Fe2+-O > Na+-O > Mg2+-O > Ca2+-O > Li+-O
This is probably, at least in part, due to the effective ionic radii (which would affect the bond length of each cation-anion bond).
Young (1961) summarized the work of other researchers that found that the A–X distance, i.e. the length of a cation-anion bond, and Mohs hardness are related for oxides with similar structures. He also states that the increase in valency of ions causes an increased hardness. This shows good agreement with the findings of Cameron et al. (1973) above.
Dana and Ford (1932) postulate that many oxides and silicates have a higher hardness because they contain significant Al. Indeed, corundum (Mohs 9) and topaz (Mohs 8) both contain aluminum as a major component of their chemistry. This supports the notion that bond length and valency play a major role in determining the hardness of a material.
There is a good correlation between the above studies and our findings that the hardness of fei cui minerals seem to increase with the increase of kosmochlor (NaCr3+Si2O6) and jadeite (NaAl3+Si2O6) contents and decrease in hardness with increased omphacite (containing more Ca2+ + Mg2+ + Fe) content.
However, hardness (and scratch resistance) is only one of the factors influencing the durability of gem materials. Toughness, i.e. resistance to breakage, is another major factor. Jade is notoriously tough and this is mainly due to the grains stopping excessive fracture continuation (Bradt, Newnham & Biggers, 1973). This makes jade an excellent carving material. Even though nephrite has a lower hardness than jadeite, its toughness is superior (Hughes et al., 2000).
HARDNESS IN RELATION TO QUARTZ
It has been stated by Schluessel (in Jade: A Gemologist's Guide edited by Hughes, 2022) that the hardest component of dust that jewelry would be exposed to is quartz and because quartz has a higher hardness than jadeite, wiping the dust off of jadeite will slowly damage its surface. The composition of global dust has been studied by Claquin, Schulz and Balkanski (1999), Krueger (2004) and Laskina (2013) who found that the major components of global dust are clays, calcium-rich minerals (such as dolomite and calcite) and quartz. The exact composition varies depending on the dust source. The hardest of these is indeed quartz. It also, therefore, makes sense to compare the hardness of quartz to that of the fei cui samples.
Many of the fei cui samples from this study were deformed (some to lesser degrees than others) by a Mohs 7 quartz-tipped pencil. Therefore, Schluessel's advice on caring for fei cui is valid. However, because the scratches are only on a microscopic scale, it is unlikely that any fei cui gems are at severe risk of damage if stored properly.
There are some examples of expensive gems (i.e. tanzanite and opal) that do not have a Mohs hardness greater than quartz, but that does not mean their value is depreciated; they just require extra care.
Hänni, Brunk and Franz (2021) investigated the grinding hardness of their jadeite sample (that contained 35% chromian omphacite) and found it to have a grinding hardness less than many of the quartz varieties they tested, but not all of them. This highlights the difficulty in trying to comprehensively assess the hardness of a gem material, especially those of a polycrystalline/polymineralic nature.
CONCLUSION
There is no easy answer to the question "is one fei cui mineral harder than the other?"; the results detailed in this paper highlight the difficulty in assessing the hardness of fei cui and the differences between hardness and scratch resistance. Some fei cui samples that, based on the available testing data, contain higher amounts of omphacite seem to exhibit a lower hardness and scratch resistance than those with lower omphacite contents. However, due to the difficulty in accurately, and non-destructively, determining the omphacite content of fei cui gems, it would be superfluous to expect the gem trade to have to separate omphacite-rich fei cui jade based on the fear that they might be slightly less scratch resistant than jadeite- or kosmochlor-rich fei cui jade. There are other factors to consider when assessing the durability of a gem; fei cui and nephrite are both extremely tough materials. Nevertheless, it would be wise to treat all jade with the same care.
It would not be prudent to label a piece of pyroxene jade as "jadeite" as it is not scientifically defensible to do so. "Jadeite" is an end-member of the clinopyroxene group defined by its specific chemical composition and structure. Currently, no laboratory (gemological or otherwise) can ascertain the exact composition of a piece of jade in relation to its end-members (and other constituents) without destroying the sample.
Mineralogy and gemology are related fields, but serve different purposes; the former is purely scientific where the latter serves the gem and jewelry trade by connecting science and commerce. Indeed, one of the most difficult parts of gemology is to find the correct balance between science and practicality. Ultimately, the end-consumer needs to know the (gemological) identification of the gem which they are buying, whether it is natural or synthetic and whether it has been treated in any way. Trying to describe a piece of jade as, for example: 65% jadeite, 20% omphacite and 15% kosmochlor is cumbersome (let alone not practically possible without destroying the gem) and would create unnecessary distress in the trade. The term "fei cui" is historically accepted by the largest consumers of the material and is both scientifically defensible and practical. As our knowledge of gems evolves, so too must our classification.
To accommodate this, all the gemological reports issued by Lotus Gemology since 1 July 2023 replace the term jadeite with "fei cui." Future editions of Jade: A Gemologist's Guide will do the same.
 Sample Lotus Gemology report for fei cui jade, showing the wording that appears on the report. Click on the image for a PDF of the full report.
Sample Lotus Gemology report for fei cui jade, showing the wording that appears on the report. Click on the image for a PDF of the full report.
|
Appendix A • Mineralogical vs. Gemological Nomenclature Despite the fact that gemology incorporates a healthy slice of mineralogy (as most gems are minerals), the two fields are actually quite different. Mineralogy is a subset of physical science and as such, must ultimately answer to scientific truth. That "truth" will necessarily change as scientific knowledge changes, but there are strict definitions and a general agreement as to how those standards are applied. When it comes to the names of minerals, the International Mineralogical Association (IMA) sets the standards on what does and does not qualify as a legitimate mineral species and the world mineralogical community generally accepts the findings. Gemology is actually a field in service of the sale of a group of commerical products, and thus must ultimately answer to commercial interests. While attempts have been made to standardize gemological nomenclature (think AGTA, ICA, CIBJO), due to variations in culture, local conditions and laws, standardized nomenclature has never taken hold. Which brings us to jade. Scientifically, the term "jade" is completely unsupported, as it is used to describe gem materials from two entirely different mineralogical groups (amphiboles and pyroxenes). And yet the term has existed for thousands of years and has huge commerical importance. But when gemologists attempt to subdivide jade into mineral species/subspecies, they crash into a scientific dead end. Current mineralogical nomenclature involves the following definitions:
Simplified mineralogical hierachy nomenclature looks something like this:
Which brings us back to jade. Mineralogically speaking, "jadeite" has a specific definition based on its chemical composition and structure. If you have an aggregate material composed of two or more intermixed mineral species, convention is to name the overall mineral based on which species is dominant. But if you have an aggregate of discrete crystals and are unable to accurately determine the overall chemical composition/structure, it is not just disingenuous to give it a single species name, but scientifically unsupportable. |

POSTSCRIPT
Dr. Edward Liu of Hong Kong delivered a superb lecture entitled "Jadeite Jade or Fei Cui?" in cooperation with Rui Galopim de Carvalho and Edward Johnson. It is available on YouTube.com at the link below. The link begins at roughly the 30-minute mark, where Edward Liu's portion begins.
REFERENCES
- Adamo, I., Pavese, A., Prosperi, L., Diella, V., Ajò, D., Dapiaggi, M., Mora, C., Manavella, F., Salusso, F., & Giuliano, V. (2006) Characterization of omphacite jade from the Po valley, Piedmont, Italy. Journal of Gemmology, Vol. 30, No. 3/4, pp. 215–226.
- Ashby, N.A. (1951) The factor of hardness in metals. New Zealand Engineering, Vol. 6, No. 1, pp. 33–34.
- Bradt, R.C., Newnham, R.E., & Biggers, J.V. (1973) The toughness of jade. American Mineralogist, Vol. 58, No. 7/8, July–August, pp. 727–732.
- Brenker, F.E., Prior, D.J., & Müller, W.F. (2002) Cation ordering in omphacite and effect on deformation mechanism and lattice preferred orientation (LPO). Journal of Structural Geology, Vol. 24, No. 12, pp. 1991–2005.
- Broz, M.E., Cook, R., & Whitney, D.L. (2006) Microhardness, toughness and modulus of Mohs scale minerals. American Mineralogist, Vol. 91, No. 1, January, pp. 135–142.
- Cameron, M., Sueno, S., Prewitt, C.T., & Papike, J.J. (1973) High-temperature crystal chemistry of acmite, diopside, hedenbergite, jadeite, spodumene, and ureyite. American Mineralogist, Vol. 58, No. 7/8, July–August, pp. 597–618.
- Clark, J.R., & Papike, J.J. (1968) Crystal chemical characterization of omphacites. American Mineralogist, Vol. 53, No. 5/6, pp. 840–868.
- Claquin, T., Schulz, M., & Balkanski, Y. (1999) Modeling the mineralogy of atmospheric dust sources. Journal of Geophysical Research, Vol. 104, No. 18, pp. 22243–22256.
- Craig, J.R., & Vaughan, D.J. (1994) 'Quantitative Methods—Microindentation Hardness', in Ore Microscopy and Ore Petrography. John Wiley & Sons: USA, pp. 106–119.
- Dana, E.S., & Ford, W.E. (1932) A Textbook of Mineralogy. 4th edition. John Wiley & Sons: New York, 851 pp.
- Deer, W.A., Howie, R.A., & Zussman, J. (1992) An Introduction to the Rock-Forming Minerals. 2nd edition. Pearson: United States, 696 pp.
- Dorner, D., & Stöckhert, B. (2004) Plastic flow strength of jadeite and diopside investigated by microindentation hardness tests. Tectonophysics, Vol. 379, pp. 227–238.
- Franz, L., Sun, T.T., Hänni, H.A., Capitani, C., Thanasuthipitak, T., & Atichat, W. (2014) A comparative study of jadeite, omphacite and kosmochlor jades from Myanmar, and suggestions for a practical nomenclature. Journal of Gemmology, Vol. 34, No. 3, pp. 210–229.
- Gemmological Association of Hong Kong (GAHK) (2016) Standard Methods for Testing Fei Cui for Hong Kong. Hong Kong: Gemmological Association of Hong Kong, unpaginated (~46 pp.).
- Gerberich, W.W., Ballarini, R., Hintsala, E.D., Mishra, M., Molinari, J., & Szlufarska, I. (2015) Toward demystifying the Mohs hardness scale. Journal of the American Ceramic Society, Vol. 98, No. 9, pp. 2681–2688.
- Hänni, H.A., Brunk, R., & Franz, L. (2021) An investigation of grinding hardness of some ornamental stones. Journal of Gemmology, Vol. 37, No. 6, pp. 632–643.
- Hansford, S.H. (1948) Jade and the kingfisher. Oriental Art, Vol. 1, No. 1, pp. 11–17.
- Hernández-Murillo, C., García-Piedra, S., Alfaro-Córdoba, M., Fernández-Esquivel, P., Ménager, M., & Monteroa, M.L. (2022) Influence of surface roughness on the spectroscopic characterization of jadeite and greenstones archaeological artifacts: The Axe-God pendants case study. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, Vol. 267, No. 1, unpaginated (~33 pp.).
- Htein, W., & Naing, A.M. (1995) Studies on kosmochlor, jadeite and associated minerals in jade of Myanmar. Journal of Gemmology, Vol. 24, No. 5, pp. 315–320.
- Hughes, R.W., ed. (2022) Jade: A Gemologist's Guide. Lotus Publishing: Bangkok, 534 pp.
- Hughes, R.W., Galibert, O., Bosshart, G., Ward, F., Oo, T., Smith, M., Sun, T.T., & Harlow, G.E. (2000) Burmese jade: The inscrutable gem. Gems & Gemology, Vol. 36, No. 1, pp. 2–26.
- Hutchinson, C.S. (1974) Laboratory Handbook of Petrographic Techniques. Wiley-Interscience: New York, 527 pp.
- Krueger, B.J., Grassian, V.H., Cowin, J.P., & Laskin, A. (2004) Heterogeneous chemistry of individual mineral dust particles from different dust source regions: the importance of particle mineralogy. Atmospheric Environment, Vol. 38, pp. 6253–6261.
- Lawn, B.R., & Marshall, D.B. (1979) Hardness, toughness, and brittleness: An indentation analysis. Journal of The American Ceramic Society, Vol. 62, No. 7/8, pp. 347–350.
- Laskina, O., Young, M.A., Kleiber, P.D., & Grassian, V. H. (2013) Infrared extinction spectroscopy and micro-Raman spectroscopy of select components of mineral dust mixed with organic compounds. Journal of Geophysical Research: Atmospheres, Vol. 118, pp. 6593–6606.
- Lenzen, G. (1970) The History of Diamond Production and the Diamond Trade. Trans. by Bradley, F., London: Barrie and Jenkins, 1st English edition, 230 pp.
- Liu, S.I., Ou Yang, C.M., & Ng, F.Y. (2015) The application of VPSEM-Raman coupled system in studying fei cui. 34th International Gemmological Conference IGC: Vilnius, Lithuania, pp. 76–79.
- Matsumoto, T., Tokonami, M., & Morimoto, N. (1975) The crystal structure of omphacite. American Mineralogist, Vol. 60, No. 7/8, July–August, pp. 634–647.
- Morimoto, N., Fabries, J., Ferguson, A.K., Ginzburg, I.V., Ross, M., Seifert, F.A., Zussman, I., Aoki, K & Gottardi, G. (1988) Nomenclature of pyroxenes. Mineralogical Magazine, Vol. 52, No. 9/10, September–October, pp. 535–550.
- Mukhopadhyay, N.K., & Paufler, P. (2006) Micro- and nanoindentation techniques for mechanical characterisation of materials. International Materials Reviews, Vol. 51, No. 4, pp. 209–245.
- Murray, J.A.H. (1900) The etymology of jade (the mineral). Athenæum, Vol. 2, October 20, p. 513.
- Ou Yang, C.M., Li, J.Q., Li, H., & Kwok, B. (2003) Recent studies on inky black omphacite jade, a new variety of pyroxene jade. Journal of Gemmology, Vol. 28, No. 6, pp. 337–344.
- Petrik, J. (2016) On the load dependence of micro-hardness measurements: Analysis of data by different models and evaluation of measurement errors. Archives of Metallurgy and Materials, Vol. 61, No. 4, pp. 1819–1824.
- Sargent, P.M., & Page, T.F. (1978) The influence of microstructure on the microhardness of ceramic materials. Proceedings of the British Ceramic Society, Vol. 26, pp. 209–224.
- Sawamura, S., & Wondraczek, L. (2018) Scratch hardness of glass. Physical Review Materials, Vol. 2, No. 9, pp. 1–5.
- Schluessel, R. (2022) "Caring for Jade", in Hughes (2022) Jade: A Gemologist's Guide. Lotus Publishing: Bangkok, p. 365.
- Shi, G., Harlow, G., Wang, J., Wang, J., Ng, E., Wang, X., Cao, S., & Cut, W. (2012) Mineralogy of jadeitite and related rocks from Myanmar: A review with new data. European Journal of Mineralogy, Vol. 24, pp. 345–370.
- Tabor, D. (1954) Mohs's hardness scale – A physical interpretation. Proceedings of the Physical Society, Vol. 67, No. 3, pp. 249–257.
- Tan, L., Lee, C.W., Chen, C., Tien, P., Tsui, P., & Yui, T. (1978) A Mineralogical Study of the Fentien Nephrite Deposits of Hualien, Taiwan. NSC Special Publication No. 1. National Science Council: Republic of China, 81 pp.
- Tan, T.L., Ng, L.L., & Lim, L.C. (2013) Studies on nephrite and jadeite jades by Fourier Transform Infrared (FTIR) and Raman spectroscopic techniques. Cosmos, Vol. 9, No. 1, pp. 47–56.
- Voyiadjis, G.Z., & Yaghoobi, M. (2017) Review of nanoindentation size effect: Experiments and atomistic simulation. Crystals, Vol. 7, No. 321, pp. 1–28.
- Young, B.B. (1961) The Microhardness of Opaque Minerals. Ph.D. thesis. University of London, 509 pp.
ABOUT THE AUTHOR
Kaylan Khourie is a South African gemologist with almost a decade of laboratory experience focusing on diamond, corundum, beryl, tanzanite and many other gemstones. A Fellow of the Gemmological Association of Great Britain (FGA) since 2017, Kaylan has since gained a special interest in unique gems, rare synthetics and abnormal treatments. He is a big football fan and enjoys spending quality time with his family. Kaylan joined Lotus Gemology in early 2023.
ACKNOWLEDGEMENTS
The author wishes to acknowledge Lotus Gemology's Richard Hughes, Wimon Manorotkul, Billie Hughes, Sayomphu Jeerat, Chanon Yimkeativong and Ronnakorn Manorotkul for the assistance in several aspects of preparing this article. Thank you to Edward Liu for permission to use the BSE images, donation of the kosmochlor sample and review of the article. Thanks also to Tom Banker, Jason C.H. Kao and Steve Ulatowski for sample donations. A special thank you to my wife Taryn for the support and assistance with formulating the data visualisations.
Notes
First published in the Journal of the Gemmological Association of Hong Kong (2023), Vol. 44, pp. 45–57. This online version contains additional material not found in the print version.