The early history of beryllium diffusion in sapphire, starting with the 2001 appearance of large numbers of padparadscha sapphires in the Thai gem market.
March 2002 – In October 2001, Australian gemologist Terry Coldham informed the author of a new treatment for orange sapphire. His initial report was that a burner in Chanthaburi, Thailand had developed a new method to treat off-color Songea (Tanzania) stones to a fine orange to red-orange color. Shortly thereafter, several other sources confirmed the news. The stones were to be marketed under new names, such as Sunset sapphire, etc.
Following the author's email blast with the news provided him by Coldham, AGTA Lab Director Ken Scarratt visited Bangkok in December 2001 and obtained samples. Pala International's Bill Larson also purchased samples in early December 2001 in Bangkok. When Scarratt examined his stones back in New York, he found that all had been exposed to high-temperature heat treatment. But under immersion, many displayed unusual orange color rims surrounding pink cores, suggesting there might be more to this than a simple bake job.
On December 28, 2001, Scarratt asked the author if he knew anything about the stones, mentioning the orange color rims. We quickly examined the Pala stones just purchased in Bangkok and found identical color rims on most pieces. Shortly thereafter, the AGTA issued a Lab Alert and Pala International sent a number of stones to the GIA for analysis. The following is based upon the AGTA Report of Jan. 8, 2002 and GIA reports of Jan. 28, 2002 and Feb. 16, 2002, along with discussions we have had with American experts, such as John Emmett, [1] and other dealers and gemologists around the world. [Note: new online reports by the GIA and AGTA were issued on April 19, 2002, with further updates on May 3, 2002 and 5 Sept., 2002]
Color Ranges and Types
The finished color range of these goods runs the gamut from yellow through golden yellow, to orange (including the range that encompasses padparadscha) and even into borderline ruby colors, some of which resemble red spinels. What initially began as a treatment for Songea sapphires quickly spread to Madagascar pink sapphires, off-color Thai/Cambodian rubies and even green sapphires from Australia and elsewhere.
From what we can gather so far (and the situation is changing rapidly), pink Madagascar stones are treated to orange (including padparadscha colors), green sapphires from Songea, Thailand and Australia are being treated to golden colors, and off-color Songea orange and reddish sapphires and purplish Thai/Cambodian rubies treated to better, redder colors. The following report applies only to stones that show orange color rims.
Diffusion Treated? Likely.
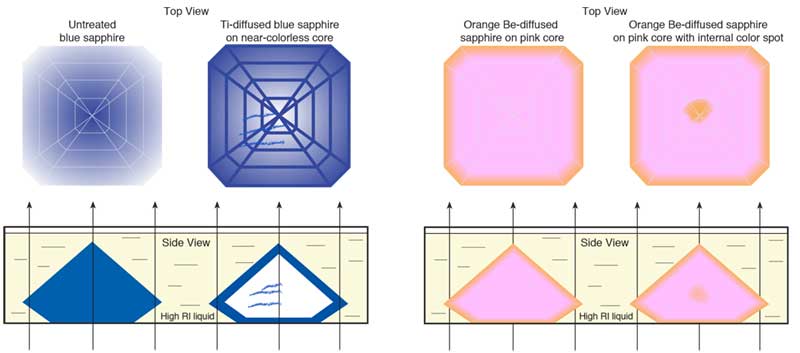
An unusual characteristic in many stones is a surface-based orange color layer surrounding a pink core (see photo below). Superficially, this resembles surface (bulk) diffusion (see box below), but unlike previous surface-diffusion treated gems, the facet junctions and girdle show no highlighting. Instead, what is seen is a layer of yellow-orange that follows the shape of the cut stone exactly. This suggests that at least the final portion of the treatment is applied to the cut stone, rather than the rough. It also suggests something being added from outside, because if it was simply a heat treatment acting upon elements already within the stone, the internal color pattern would not follow the shape of the cut stone exactly. There is no mine that produces rough orange sapphires in a perfect trillion shape (see below).
 No, this gem does not come from the "trillion" mine, but is a Madagascar pink sapphire with an orange color rim created by bulk diffusion. The gem is immersed in di-iodomethane. Note that recutting such stones will produce a loss of the orange color. Photo: R.W. Hughes
No, this gem does not come from the "trillion" mine, but is a Madagascar pink sapphire with an orange color rim created by bulk diffusion. The gem is immersed in di-iodomethane. Note that recutting such stones will produce a loss of the orange color. Photo: R.W. Hughes
In addition to the now-common orange rinds on orange sapphires, sources have reported similar orange rims on rubies. Indeed, one told the author that burners in Thailand are now actively seeking off-color Thai/Cambodian rubies for color improvement via the added orange color rind. Similarly, those burners are said to be seeking old stocks of green sapphires for treatment to golden colors.
Recutting? Just say "no."
We attempted to recut one emerald-cut orange stone, but stopped after just 0.12-ct. of weight loss when serious color loss was noted. Another source reported a similar loss of color during recutting. In other stones, the color appears to go all the way through. Dealers have reported recutting these color rind-free stones with little or no loss of color.
Golly, Molly, what are these things?
To answer that question, a meeting was held in Tucson on Feb. 5, 2002. In attendance were gemologists and dealers from around the world, including representatives from Thailand. Theories discussed included the following:
Zap Mama?
Initial reports suggested stones were possibly undergoing irradiation and that the color was unstable, fading with prolonged light exposure. However, the GIA's Shane McClure pointed out that such irradiation would not color an entire stone's surface equally, which is what appears with many of these new stones. Reports on fade tests have also resulted in no loss of color. Thus we can safely scratch irradiation as a possibility.
Geritol-rich gems?
Thai-based online reports pointed to an alteration of the valence state of iron from Fe2+ to Fe3+ as the possible cause of the orange rims.
Discussions here in America suggest this is not the case. According to John Emmett, former Associate Director for Lasers at Lawrence Livermore National Laboratory, and one of the world's top experts on the chemistry and physics of corundum, for iron to produce a yellow color in corundum, iron substitutions on the order of 2–3% are required. To the best of our knowledge, this is not being found in the pinkish orange stones with color rims.
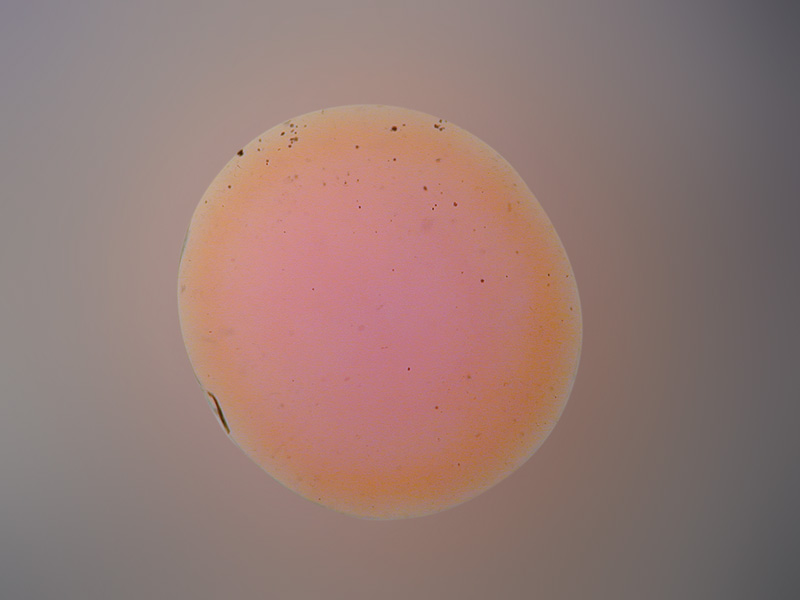 Orange rim surrounding a pink core in a bulk-diffusion treated orange sapphire from Madagascar. The color rim is visible when the gem is immersed in di-iodomethane and is evidence of a treatment applied after cutting. Note that recutting such stones will produce a loss of the orange color. Photo: R.W. Hughes
Orange rim surrounding a pink core in a bulk-diffusion treated orange sapphire from Madagascar. The color rim is visible when the gem is immersed in di-iodomethane and is evidence of a treatment applied after cutting. Note that recutting such stones will produce a loss of the orange color. Photo: R.W. Hughes
Burned at the steak?
Yet another theory is that the stones are cooked "like a steak." Gems with shallow color rims are equivalent to "medium rare" cooking while those with darker colors where the color goes all the way through are "well done."
Again, John Emmett dismissed it, stating that corundum is essentially "isothermal," meaning that it conducts heat so well that there is no significant temperature difference between the skin and center of a gem. Of the major gems, only diamond and silicon carbide have better thermal conductivity than corundum.
Synthetic overgrowth?
Perhaps the most bizarre theory on these color rims was that of Themelis.com, which in a briefly aired report suggested that they represented synthetic overgrowths of orange sapphire on pink sapphire cores. No evidence of this has been found. [Note: While we initially found no evidence of this, the latest AGTA report of April 19, 2002 suggests that dissolution of the gem surfaces by fluxes and redeposition of synthetic corundum may play a part in this treatment]
Showdown at the Tucson Corral
With x-ray, iron, steak and fake discredited, it was left to John Emmett to explain. At the Tucson meeting, he described the most likely cause of the orange color rims as outside-in "surface-diffusion" of a trapped-hole color center-producing ion. Such an element could be any small, light, aliovalent ion from the upper left corner of the periodic table. Likely candidates are magnesium (Mg2+), beryllium (Be2+), calcium (Ca2+), lithium (Li+), sodium (Na+) or potassium (K+). Even things like copper (Cu2+) and silver (Ag+) could be involved.
According to John Emmett, at high temperatures the diffusion process draws elements present on the surface of the stone into the stone. At the same time, when this process is conducted in an oxidizing atmosphere, point defects called "holes" (which are the absence of an electron) are also created on the surface, and they diffuse much more rapidly throughout the stone. If some of these holes are trapped by the beryllium , magnesium, etc. which has diffused into the stone, they create what is called a "trapped-hole color center." In corundum, the trapped-hole color centers create a strong yellow coloration. This yellow coloration in a stone with a pink body color creates an orange coloration. However, not all stones will react the same way during this treatment. If titanium or other tetravalent impurities are present, they can bind with the magnesium or beryllium in such a way as to prevent formation of the trapped-hole color centers. Thus the relative amounts of the diffused-in element, and all the other impurities naturally in the stone, will determine the final color. This explains why individual stones react differently to the treatment.
Further evidence of a trapped-hole color center is the nature of the color itself. According to Emmett and Douthit (1993):
- The strong orangey yellow coloration produced by this [Mg2+] point defect is very different from the pale pure yellow of iron-produced coloration.
- The quantities required to develop such color centers are infinitesimal, as little as 20–30 parts per million. But this creates a further problem. Such levels are virtually undetectable, even for well-equipped labs such as the AGTA or GIA. In the end, the actual colorant may be undetectable with current technology.
A second problem is that, again according to our current understanding, so little of the aliovalent ion may be required for this treatment that burners may not be aware that they are surface-diffusing these stones.
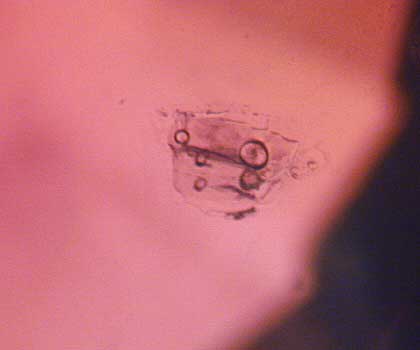 This melted crystal with trapped gas bubbles in an orange sapphire believed to be from Songea, Tanzania, is strong evidence of high-temperature heat treatment.
This melted crystal with trapped gas bubbles in an orange sapphire believed to be from Songea, Tanzania, is strong evidence of high-temperature heat treatment.
Photo: R.W. Hughes
Science to the Rescue
At the suggestion of John Emmett and Intel's Gene Meieran, just before Tucson the GIA subjected three different cross-sectioned samples to Laser Ablation Inductively Coupled Mass Spectrometry (LA-ICP-MS) and Secondary Ion Mass Spectrometry (SIMS) analyses. The GIA's Shane McClure presented the results of the tests at the Tucson meeting.
While LA-ICP-MS turned up nothing unusual, SIMS analysis revealed unusually high amounts of beryllium (Be) in the orange color layer. Since Be is not normally found in corundum, and since the elevated Be levels of the skin were not found in the pink cores of the tested samples, the evidence is quite strong that, at least in some samples, the skin color appears to be due to surface diffusion of light aliovalent ions to create a yellow-producing trapped hole color center.
Better Living Through Chemistry?
While the jury is still out on stones where the color goes all the way through (generally golden and rich red-orange in color), those with yellow-orange color rims appear to owe at least part of their face-lifts to a form of surface diffusion.
By the end of the meeting, the gathered masses had divided into two camps:
- Dealers who held stocks of such goods, along with gemologists who had issued heat-only reports on such goods
- Everyone else
Face-saving measures are now under discussion. Some have suggested the stones simply be referred to as "treated," with a mention about their surface-based coloration. But as former AGTA President, Owen Bordelon stated at the Tucson meeting: "People are seriously deluded if they think these stones will fly off dealers' shelves, even with such a description."
An additional problem is that some labs and schools have been mistakenly referring to surface-diffusion treated sapphires as simply "diffusion treated."
Do we need new nomenclature for these stones? I don't think so. Why should we invent a new name just because certain stones were initially misidentified or because some used incorrect terminology? Properly used, our current nomenclature will suffice.
According to Emmett, these new stones (those with well defined surface-conforming color rims), are in no way different than the blue surface-diffused (Ti) stones of the past. With the blue sapphires, blue was diffused into colorless as well as unevenly-colored blue material. Note that blue surface diffusion also requires the presence of naturally-occurring iron in the stone to react with the inward diffusing titanium to produce a the blue coloration. Given the similarities between the blue surface diffusion of old and the orange surface diffusion of today, logically the descriptions should be parallel.
 Bulk-diffusion treated orange sapphires of the type described above, purchased in Bangkok by Pala International in Dec., 2001. Photo: Robert Weldon
Bulk-diffusion treated orange sapphires of the type described above, purchased in Bangkok by Pala International in Dec., 2001. Photo: Robert Weldon
In solid-state physics, that which we gemologists term "surface diffusion" is referred to as "bulk" or "lattice" diffusion. This separates outside-in movement of light elements like hydrogen from similar outside-in movement of heavier elements like titanium, chromium and magnesium. It's not a question of how deep the penetration, but more a question of what is going in.
The use of the term "surface diffusion" in gemological nomenclature is an attempt to separate treatments that influence colorants already within a gem from those that introduce new colorants from outside. This relates to rarity, because treatments that depend on colorants already within the gem are limited in the changes they effect.
In contrast, treatments that involve outside-in movement of coloring agents (surface or bulk diffusion) have far more leeway in the changes they can effect. Given a gem canvas that is relatively pure and light in color, treaters can theoretically paint color at will. This begs the question: when human intervention becomes such a large part of a gem's apparent quality, why mess around? Why don't treaters just get busy producing a full synthetic?
Deep down inside, we all know the answer to that one.
Prickly heat
Over the past twenty years, a number of controversies similar to this have occurred in our trade. In the late 1970's and early 1980's, it was the appearance of heat-treated rubies and sapphires (Hughes, 1995). Producers originally sold them as completely natural. When it became understood that the stones had been heated, they fought tooth and nail to avoid the disclosure of those treatments. Today disclosure is the norm.
In the early 1980's, the first surface-diffusion treated blue sapphires appeared. Producers initially sold them as natural, later as simply heated. Today, full disclosure is the norm.
The mid-1980's saw Thai/Cambodian rubies with glass-filled surface cavities appear (Hughes, 1984). Again, initially sold as natural. Today, full disclosure is the norm.
By the late 1980's, a second-wave of surface-diffused stones appeared, with just a little color added on already blue stones with color zoning problems (Hughes, 1988, 1991, 1992). Producers initially denied the treatment, stating that stones had received only "surface heating." The world's labs did not accept this explanation. Today, disclosure is the norm and, in this particular case, such stones have largely disappeared from the market. [2]
In the early 1990's, rubies from Möng Hsu appeared. Originally they were sold as simply heated. When glassy residues were found, producers stated this was just a byproduct of heating. It was later shown that such stones were deliberately heated in fluxes to heal their fractures with what amounts to synthetic ruby (Hughes and Galibert, 1998; Emmett, 1999; Hänni, 2001). Even today, some try to deny what is done to these stones, while many others do not fully understand it. But disclosure is becoming the norm.
Later in that decade, emerald oiling became an issue of controversy (Hughes, 1998, 2000). Producers and even some CIBJO members fought vigorously for over a decade to avoid disclosure. Today, disclosure is the norm.
Flash forward. 2002. Once again, we have a new treatment. And we are being told it involves one thing, while the evidence indicates another.
Déjà vu.
Based on the historical record and current evidence, it appears only prudent to go slow with these stones.
Future Games
A man's eyes should be torn out if he can only see the past. Russian proverb
In the end, attempting to equate a treated gem with one created by nature is a mistake. For far too long we in the colored stone business have tried to gloss over the difference. It is time we stopped trying and began admitting that there is a huge difference between a natural stone and an artificially enhanced product. If we value the natural product – if we wish it to survive in the marketplace – the only chance it stands is with full disclosure of all treatments.
Eight years ago I wrote the following:
Gem enhancements will not become any less effective, nor will detection become easier. Such a clever cat, the trade asked for a better mousetrap, but now complains because all the mice are dead and it has nothing to eat.
We used to believe in magic. We thought that everyone could get rich by making silk purses out of sows' ears. But we failed to see into the future. We rubbed the magic lamp, the enhancement genie appeared, but now he's turned on his master. And suddenly we've decided that we don't believe in magic after all.
I still believe in magic. I still remember the magic that holding a fine Burma ruby first brought. Today my daughter is five years old. I hope that when she is my age, she still believes in magic. I hope that when she holds a fine gem, she holds a silk purse, not a sow's ear.
Maybe it's that time again – time to reconsider what the future might hold.

|
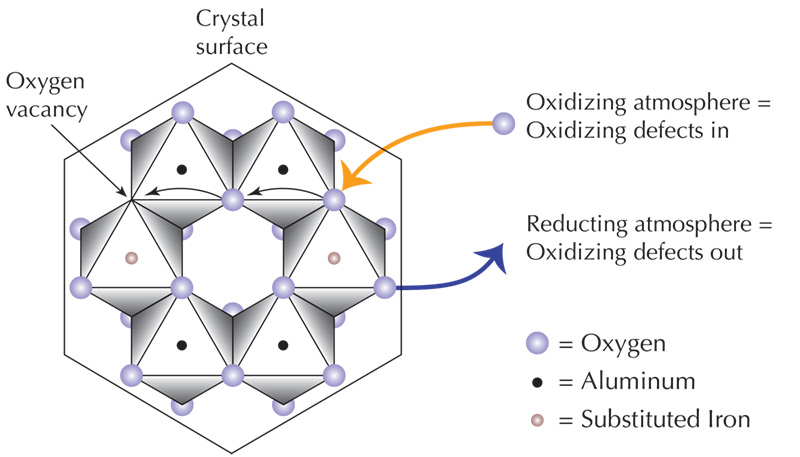
Notes on Diffusion in Corundum For the following description of diffusion in corundum, we thank John Emmett of Crystal Chemistry, Brush Prairie, WA. But please note that the account should be thought of as preliminary only, since Mr. Emmett has not written this himself. In other words, all mistakes are mine, not those of Dr. Emmett. Over the years, a number of claims have been made regarding heat treatment. Perhaps the most common is that "nothing is added" during heat treatment. Current scientific evidence simply does not support this idea. During the heat treatment of corundum, elements and subatomic particles shift both state and position, including atoms moving into the gem from outside. Alteration of the valence states of impurity atoms is particularly important. The latter is done via diffusion (movement) of hydrogen (H) or aluminum (Al) vacancies in or out of a stone. Even though it might seem like nothing, hydrogen is a chemical. You find it on the periodic table of the elements, just like titanium (Ti), chromium (Cr), nickel (Ni) and vanadium (V). Corundum is made up of Al2O3, with the aluminum in the Al3+ valence state. Impurity elements with the same valence, such as Cr3+, Fe3+ and V3+ are termed isovalent and will easily and happily substitute for Al3+ in the corundum structure. These elements require substitutions in the 0.1–3% range to produce significant color in corundum. Another type of coloration, however, is caused by color centers and requires far smaller impurity amounts. In these cases, aliovalent ions, meaning those with a different valence from the ion they replace, are involved. Examples include Mg2+ and Ti4+. When an aliovalent ion replaces Al3+, the fit is not so happy, and defects are created which absorb light. The most important situation is where lower valence ions (such as Mg2+) are present in larger quantities than higher valence ions (Ti4+). When this requirement is met, yellow-producing trapped-hole color centers can result. Now we come to the good part. Heating a gem in the presence of oxygen can activate such color centers in corundum. Virtually any light-colored Sri Lankan sapphire can be heated to produce a yellow to golden color in an oxidizing atmosphere because these stones naturally possess the Mg2+ needed. In the case of Montana sapphires (Rock Creek, Missouri River), the Mg2+ is often present in greater concentrations in the core of the crystal. Thus heating in an oxidizing atmosphere causes a deep yellow core in many stones. Heating a pink stone may add the yellow-producing color centers to the pink color, causing a padparadscha-like color, if the above conditions are met. Heating a purple stone adds yellow to the purple, thus producing a redder, more ruby-like stone. Heating a blue stone adds yellow to the blue, which is not normally done because it muddies the blue. But what if your stone does not have the needed ion? You can diffuse it in from the outside. While diffusion rates for isovalent ions such as Cr3+, Fe3+ and V3+ are agonizingly slow, aliovalent elements such as Mg2+ and Ti4+ diffuse into corundum some 1000–10,000 times faster. Thus it is possible to add significant color to corundum, via diffusion of tiny quantities of aliovalent ions from outside. How much is needed? It may be as little as a few tens of parts per million. Such traces are virtually undetectable with the types of instruments found in even the most well equipped gemological labs. In some cases, burners may be diffusing aliovalent ions into their stones without even realizing it. Indeed some fluxes commonly used in burning (such as borax) may contain traces of Mg2+. Other aliovalent ions that can possibly produce this effect include lithium, beryllium and sodium. Dr. Emmett has reproduced these diffusion experiments in his laboratory. In some cases, he found that traces of Mg2+ contamination enough to produce some coloration were found even in the highly purified alumina from the crucibles. Post-burn yellow or cream coloration of a previously snow-white alumina crucible may be evidence of such contamination. When Ti4+ is diffused into corundum to produce a blue color, it moves in quickly until the point where it meets Fe ions. At that point, bonds are formed with Fe and the diffusion generally ceases. This is why we often find a sharp blue boundary between a diffused zone and the colorless core in surface-diffusion treated blue sapphires. Dr. Emmett has done controlled burns of Ti surface diffusion with sapphires of varying Fe contents that confirm this effect. Penetration of blue color is more diffuse in stones of lower Fe content. With stones of higher Fe content, a sharper boundary is found between the blue diffusion skin and the crystal core. With Mg2+, however, no such bonding with iron occurs. The result is that Mg2+ diffusion into corundum does not produce the same sudden color transitions and darkened facet junctions as those found with blue Ti4+-based diffusion. All this theory goes a long way towards explaining the types of things we have been seeing over the past few weeks with these new orange sapphire treatments. Burners may be burning stones with nothing other than oxygen and if a stone naturally possesses enough of the proper impurity, it may develop an orange color. In some stones, where the impurity is present only in one area, just that area will turn yellow (or orange). With other stones, the impurity may be diffused in from outside, via a flux or other impurity present during the burn. And in other cases, we may see a combination effect, where some impurity is diffusing in from outside, along with an activation of impurities already present in the crystal. Further details of the diffusion process can be found in Chapter 6 of my 1997 book, Ruby & Sapphire. Much of that material is based on discussions with John Emmett. Thus I would like to again thank him for his help.
|

About the author
Richard W. Hughes is one of the world’s foremost experts on ruby and sapphire. The author of many books and over 170 articles, his writings and photographs have appeared in a diverse range of publications, and he has received numerous industry awards. Co-winner of the 2004 Edward J. Gübelin Most Valuable Article Award from Gems & Gemology magazine, the following year he was awarded a Richard T. Liddicoat Journalism Award from the American Gem Society. In 2010, he received the Antonio C. Bonanno Award for Excellence in Gemology from the Accredited Gemologists Association. The Association Française de Gemmologie (AFG) in 2013 named Richard as one of the Fifty most important figures that have shaped the history of gems since antiquity. In 2016, Richard was awarded a visiting professorship at Shanghai's Tongji University. 2017 saw the publication of Richard and his wife and daughter's Ruby & Sapphire • A Gemologist's Guide, arguably the most complete book ever published on a single gem species and the culmination of four decades of work in gemology. In 2018, Richard was named Photographer of the Year by the Gem-A, recognizing his photo of a jade-trading market in China, while in 2020, he was elected to the board of directors of the Accredited Gemologists Association and was appointed to the editorial review board of Gems & Gemology and The Australian Gemmologist magazine. In 2022, Richard published Jade • A Gemologist's Guide, while 2024 brought Broken Bangle • The Blunder-Besmirched History of Jade Nomenclature. His jade trilogy was completed in 2025 with his translation of Heinrich Fischer's Nephrite and Jadeite.
Acknowledgments
This article could not have been written without the generous help of the following (in alphabetical order): Jeff Bilgore, Edward Boehm, Kriengkrai Chiaraput, Terry Coldham, Richard Drucker, John Emmett, Josh Hall, John Koivula, William Larson, Gabrièl Mattice, Shane McClure, Gene Meieran, Yianni Melas, Roland Naftule, Karen Palmer, Visut Pisutha-Arnond, Stuart Robertson, Gary Roskin, Ken Scarratt, James Shigley, Arnold Silverberg, Mark Smith, Maha Tannous, Vichian Veerasaksri, Pornsawat Wathanakul, Robert Weldon, Ray Zajicek and Urs Zwyssig.
Afterword
First published in The Guide (2002, Vol. 21, No. 2, Pt. 1, March–April, pp. 3–7), the web edition contains material and updates not found in the print version. This was the first shot in the "Beryllium Wars." After years of meetings, heated exchanges and even threats, it was finally acknowledged by virtually all that this treatment involved bulk diffusion of beryllium into the crystal lattice. Full details are provided here:
- Emmett, John L., Scarratt, K., McClure, S.F., Moses, T., Douthit, Troy R., Hughes, R., Novak, S., Shigley, J.E., Wang, W., Bordelon, O., Kane, R.E. (2003) Beryllium diffusion of ruby and sapphire. Gems & Gemology, Vol. 39, No. 2, Summer, pp. 84–135.
Footnotes
[1] While John Emmett is not one to blow his own horn, allow me to elaborate a bit on his background. From 1975–1988, John was Associate Director for Lasers at Lawrence Livermore National Laboratory in Livermore, CA. It was here that he first began researching corundum, something that continues to this day with his own company, Crystal Chemistry, Brush Prairie, WA. While at Lawrence Livermore, the programs involved over 1500 researchers, including 300 Ph.D.'s, and in 1988 alone were funded at US$250 million. He has authored over 50 papers published in peer-reviewed scientific journals. John is considered a world authority on the physics and chemistry of corundum and has for years been involved in heat treatment.
[2] Such stones have largely disappeared because the market decided it would pay more for a heated sapphire with zoning problems than a surface-diffusion heated sapphire with no zoning.
References
A detailed description of heat treatment in corundum can be found in the following references:
- Emmett, John L. and Douthit, Troy R. (1993) Heat treating the sapphires of Rock Creek, Montana. Gems & Gemology, Vol. 29, No. 4, pp. 250–272.
- Emmett, John L. (1999) Fluxes and the heat treatment of ruby and sapphire. Gems & Gemology, Vol. 35, No. 3, pp. 90–92.
- Hänni, Henry A. (2001) Beobachtungen an hitzegehandeltem Rubin mit künstlicher Rissheilung (Observations on heat-treated ruby with artificially healed fissures). Zeitschrift der Deutschen Gemmologischen Gesellschaft, Vol. 50, No. 3, pp. 123–136.
- Hughes, R.W. (1984) Repaired surfaces found on rubies. Jewellery News Asia, No. 6, pp. 1, 33.
- Hughes, R.W. (1988) Reappearance of surface-diffusion treated sapphires in Bangkok. ICA Lab Alert, No. 12, 2 pp.
- Hughes, R.W. (1991) There's a rumble in the jungle – The sapphire face-lift face-off. Gemological Digest, Vol. 3, No. 2, pp. 17–31.
- Hughes, R.W. (1992) Devil's Advocate: Vampire blues: deep-diffusion treated sapphires. JewelSiam, No. 3, May–June, pp. 83–86.
- Hughes, R.W. (1995) A brief history of heat. Australian Gemmologist, Vol. 19, No. 2, pp. 52–54.
- Hughes, R.W. (1997) Ruby & Sapphire. RWH Publishing, Boulder, CO, 512 pp.
- Hughes, R.W. (1998) Cloak and dagger: The politics of Opticon. GQ Eye, Vol. 1, No. 1, Fall, pp. 3, 6.
- Hughes, R.W. (2000) The Digital Devil: Check Your Gut Instincts at the Door. GemKey Magazine, Vol. 2, No. 3, March–April, pp. 62–63, 104.
- Hughes, R.W. and Galibert, O. (1998) Foreign affairs: Fracture healing/filling of Möng Hsu ruby. Australian Gemmologist, Vol. 20, No. 2, April–June, pp. 70–74.